Tire Inferno
It was a fire to remember. On Oct. 31, 1983, residents of the farming community of Mountain Falls, Va., awoke to a mushroom cloud of black smoke expanding into an otherwise cloudless sky. The 300-foot-wide plume rose 4,500 feet from the floor of their valley nestled between autumn-colored ridges of the Appalachian Mountains. Some wondered whether it was an early Halloween prank gone wrong, or whether the Russians had dropped the bomb.
It was Paul and Alma Rhinehart’s tire pile. On fire.
Estimates are that the Rhinehart tire-recycling operation had handled as many as 25 million tires in the decade leading up to the fire. The enterprise sold most of the tires for retreading and for ship docking bumpers, floor mats, shoe soles, and other uses. But some 7 million tires that were in too poor condition for resale had accumulated in a pile reaching 80 feet high and strewn across some 5 acres of wooded slopes on the Rhinehart farm. Paul had a plan for these tires—to melt them down to recover and sell crude oil and scrap metal. But the fire got to the tires first.
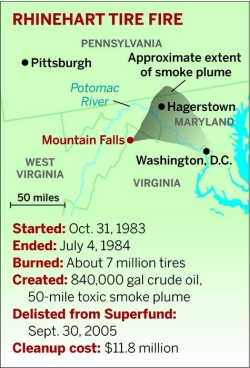
The Rhinehart tire fire ended up burning for nine months, in the process generating a plume of toxic smoke that spread across four states and a stream of thousands of gallons of crude oil from melting tires that was contained in the nick of time. The environmental disaster created by the tire fire took more than 20 years and nearly $12 million to clean up.
Tire recycling was hindered by a volume problem 30 years ago, and that hasn’t changed. According to the Rubber Manufacturers Association, one waste tire is generated per person per year in the U.S.—today that’s about 315 million tires annually.
But some things have improved. The fire served as a call to action for states to come up with better plans to manage tire recycling. The experience battling the blaze has contributed to advances in environmental monitoring. Cleaning up the fire’s toxic detritus also became one of the few success stories for the Environmental Protection Agency’s Superfund program. But perhaps the fire’s greatest legacy may be that it’s sparking start-up companies to create new technologies for using rubber and other types of feedstock materials as renewable resources.
“The fire was dramatic,” recalls Scott Mason, a photographer for the Winchester Star newspaper. Mason was at home, about 20 miles south of the fire, when he received an urgent call from an editor early that Halloween morning. “Just after dawn, the cloud of smoke looked like the dark outline of just another mountain west of Winchester,” he tells C&EN.
Instead of driving to the fire, Mason drove to the airport, where he found a pilot who was willing to take him up for a look. As he got closer, the mushroom cloud came into dramatic focus, and as Mason started to take photos he saw the wind start catching the smoke and stretching it northward—the smoke drifted for 50 miles across West Virginia and Maryland and into Pennsylvania, and also eastward toward Washington, D.C.
“It was cold up there—below freezing,” Mason continues. “I forgot to bring any gloves, so my hands were getting numb. My eyes were tearing up. I had to duck back inside the airplane window about every two shots. But I got the pictures.” One of his striking shots is on the cover of this issue.
 In 1972, at age 64, Rhinehart had started collecting and recycling tires after Frederick County, Va., banned tires from its landfill. Like many jurisdictions across the U.S., the county faced the problem that whole tires take up too much space in landfills and tend to shift underground over time. Left unburied, though, tires provide a breeding ground for mosquitoes and rats, and they can catch fire.
In 1972, at age 64, Rhinehart had started collecting and recycling tires after Frederick County, Va., banned tires from its landfill. Like many jurisdictions across the U.S., the county faced the problem that whole tires take up too much space in landfills and tend to shift underground over time. Left unburied, though, tires provide a breeding ground for mosquitoes and rats, and they can catch fire.
Rhinehart had previously been involved in car salvage operations and conceived of an idea—it’s not clear he ever got it working—to build a disassembly line to melt car parts in increasingly hot temperature zones to recover metals, using propane to burn tires for heat. Tires are made of natural (polyisoprene) or synthetic (styrene-butadiene copolymer) rubber reinforced with metal and/or fiber cords. Burning tires produces about 25% more energy than burning coal. It also produces similar pollutants, and then some.
The car recycling notion seems to have prompted Rhinehart to consider building an incinerator to smelt tires. His thinking was to drop tires into a rotating grate and let heat decompose them. Rhinehart planned to sell the oil and scrap metal and use excess heat from the incinerator to run greenhouses on his farm.
At first, Rhinehart collected tires locally, and then regionally. Eventually he started working up and down the East Coast. Travelers along I-81 through Virginia’s Shenandoah Valley may have seen Rhinehart driving a tractor-trailer truck loaded with tires, with a sign on the door that read: “Old Tire Home, Winchester, Va.”
Rhinehart was part country philosopher, tinkerer, and dreamer—a local legend—recalls Frederick County Administrator John R. Riley Jr., who often found Rhinehart standing in his office. “Mr. Rhinehart definitely had his own idea about what the role of government should be—he would have been a great Tea Party guy, no question about it,” Riley asserts. “He believed in less government, more entrepreneurship, and letting the private sector take the lead.”
But county officials grew weary of asking Rhinehart to stop hoarding the old tires and do something—to get rid of them. After he repeatedly skipped deadlines to quit, Rhinehart lost his charm with county officials. In October 1983, the county filed for a court injunction to cut him off, even as Rhinehart said he was just about ready to crank up his smelter. Days later, on the 31st, came the fire.
That afternoon, Mason, the photographer, drove out to the Rhinehart farm. By then, the place was crawling with firefighters from across the region who had been rousted out of bed to battle the blaze. Heavy flames danced across the top of the glowing tire pile, Mason recounts, but mostly the flames were shrouded by thick black smoke. Mason could see the crews were up against more than they could handle.
Smoke was a foremost concern. Scientists who study tire fires note that the smoke from burning rubber contains noxious sulfur dioxide, deadly carbon monoxide, and cancer-causing polycyclic aromatic hydrocarbons (PAHs), as well as other aromatic and nonaromatic compounds such as styrene and butadiene. “One of our employees and her husband lived in that area, and they have a lake,” Mason remembers. “The soot coming down killed all the fish.”
Behind the smoke screen, a stream of crude oil was forming deep within the tire pile. Like hot lava, the oil started dribbling out, and then later it came in torrents, as much as 200 gal per minute. An oil analysis found it contained benzene, xylenes, anthracene, and other aromatic hydrocarbons, along with some nonaromatic and sulfur compounds. The scientists detected caprolactam, a monomer for nylon fiber used in some tires, and benzothiazole, a degradation product of a compound used as a rubber vulcanizing reagent. The oil analysis also revealed arsenic, cyanide, cadmium, chromium, nickel, zinc, and other metals. The zinc would later become the biggest concern at the Rhinehart fire because its high levels would be toxic to aquatic life.
The greatest threat was the oil reaching Massey Run, a small stream adjacent to the fire, and running into Rhinehart’s farm pond, built by damming the stream. From there, the tarry ooze might reach Hogue Creek half a mile away, and from there the Potomac River 30 miles farther on. It could become a great inland oil spill.
In a Washington Post story, Kory Gabrielsen, a Virginia state hazardous materials officer, summed up the emergency situation this way: “If we don’t stop it … pretty soon it’ll be in Washington and end up in some senator’s martini.”
Firefighters quickly erected filter fences and built a catch basin to trap the oil that hadn’t seeped into the ground. Two weeks later, EPA’s emergency response team constructed a larger concrete-lined containment basin, which was named Dutchman’s Pond, in which to channel the oily waste. Some 840,000 gal of runoff would eventually be contained—most of it was deemed usable and trucked away to be refined into fuel oil. In the end, little of the flowing oil reached Massey Run.
Scientists with Virginia’s Division of Consolidated Laboratory Services analyzed burnt tire by-products in Massey Run and Hogue Creek every two weeks during the fire. The studies appear to be the only published analytical results on the Rhinehart fire.
Using gas chromatography and mass spectrometry, scientists found that only the more water-soluble compounds worked their way downstream. The most common compounds detected included caprolactam and benzothiazole, along with benzoic acid, benzonitrile, and other aromatics. The ensemble of contaminants was sufficiently high in Massey Run to be a concern. But the levels at a site 5 miles farther downstream were low enough so as not to be worrisome.
Scientists today have evolved tools to render a more exact accounting of pollutants in such disasters. Chemistry professor Elizabeth A. Stone of the University of Iowa, for example, studies natural and anthropogenic aerosol particles to assess their impacts on asthma and cardiovascular diseases, as well as on climate. Her team sets up particulate filtering units and then uses solvent extraction followed by gas chromatography and mass spectrometry to detect chemical markers in the aerosols to pinpoint pollution sources and measure pollution levels.
Stone’s group happened to have its monitoring equipment up and running over Memorial Day weekend in 2012 when a tire fire accidentally started in the Iowa City landfill.
One use of old tires today is shredding them to use as a permeable barrier layer to line landfills or as a substitute for soil cover in daily landfill operations. In Iowa City, tires had been shredded into 2- by 2-inch chips and then placed in a 3-foot-thick layer above the wastewater collection system at the bottom of a 7-acre section of the landfill. The fire, which burned an estimated 1.3 million tires and lasted a couple of weeks, produced some 150,000 gal of oil and was put out by covering the shredded tire layer with clay soil. The structural integrity of the landfill contained the Iowa City fire, Stone says, so the oil pollution doesn’t appear to have escaped into the groundwater.
But the smoke was another story. “We were able to use ambient measurements to develop an emission profile for the fire and better understand the chemical composition of the smoke and its potential health effects,” Stone explains. During the course of the fire, her team saw increases in soot (elemental carbon) and PAHs, a sign that the fire transitioned from burning vigorously to smoldering—that is, toward more incomplete combustion.
“PAH levels really shot through the roof,” Stone says. For example, at a site 2 miles from the fire, levels of highly toxic benzo[a]pyrene were up to 30 times as much as the prefire background level.
In addition to Stone’s work at the fire, atmospheric scientist Scott Spak of the University of Iowa’s Public Policy Center performed dispersion modeling, combining emissions estimates from the fire with weather forecasts to predict where the smoke plume would concentrate each day. The information was used to aid firefighting decisions and inform the public. On another front, Iowa chemical engineer Charles O. Stanier used field-deployable instruments on mobile platforms to measure gases such as carbon monoxide and sulfur dioxide and to track the evolution of the shifting smoke plume as it moved farther away from the fire. This type of monitoring wasn’t plausible 30 years ago, Stone says.
“This unexpected incident really sparked a lot of discussion and collaboration between scientists and local government,” Stone says. “It was an interesting opportunity to try to protect human health and learn as much as we can about fires to help other communities respond if something like this happens again.”
At the Rhinehart fire, the firefighting teams did the best they could to control the fire by dousing the tires with water and firefighting foam. They created fire breaks where possible and tried to safely remove tires from the burning pile. Even so, after a couple of weeks the fire still burned and the sky was still smudged with black smoke, according to newspaper reports.
Firefighting experts from around the world were consulted. They offered tried-and-true solutions for putting out oil-well fires, coal-mine fires, and landfill fires. But the interconnected array of air pockets in the haphazard pile of tires made the fire virtually inextinguishable. When a few of the experts came in person to inspect the rubber inferno, they threw their hands up. The situation looked impossible. After several months, EPA officials decided to let the fire determine its own destiny and burn itself out.
On July 4, 1984, it was Independence Day in more ways than one: The Rhinehart fire was declared dead. The pile had turned into a crusty heap prickled with the wiry remains of steel-belted radials.
Nobody seemed to know how the fire got started. Fire officials agreed that igniting the frayed Uniroyals, bald Dunlops, and other tires in the pile took more than a discarded cigarette—it would have taken high heat over an extended period or an open flame for the fire to take hold. Alma Rhinehart, quoted in the Winchester Star the day after the fire started, was certain: “Somebody set it. There’s no question about it.”
In 1987, Melvin Russell Jenkins admitted to torching the tires, although no reason was ever mentioned. Was Rhinehart involved somehow? “Probably not,” Riley says. “He wasn’t like that. Knowing the gentleman as well as I did, that would not have been his intent. He knew what the results would be.” Jenkins later was sentenced to 110 years in prison—less for the Rhinehart fire than for a string of unrelated arsons and a murder.
Rhinehart vowed that the fire wasn’t going to put him out of business. Defying the order to stop storing tires, he brought in more than 1 million more tires. Finally, in February 1991, the Virginia attorney general put a stop to the tire hoarding, citing a public health threat. Paul Rhinehart died, at age 89, on Feb. 23, 1997. Alma Rhinehart died in 2002.
The Rhinehart tire pile was not close to being the world’s largest, and the fire was not the world’s largest tire fire or the one that burned the longest. But the Rhinehart fire was 10 times as large as the biggest previous tire fire in the U.S., and it became part of EPA’s fledgling Superfund program.
Superfund is the common name given to the Comprehensive Environmental Response, Compensation & Liability Act of 1980. This law established a program to remediate an array of hazardous waste sites, including industrial plants, old junkyards, military bases, and gas stations. Superfund was created in the wake of the discovery of several severe cases of toxic waste dumping in the 1970s—Love Canal, in New York, Times Beach, Mo., and the Valley of the Drums, near Louisville, Ky., to name a few.
At the end of 2012, EPA had investigated more than 40,000 potential Superfund sites. The agency has taken action to clean up many of them, but only about 1,680 were bad enough to make it to the official Superfund list. About 370 have been cleaned up and removed from the list, and the Rhinehart tire fire is one of them.
In 1988, EPA instituted a plan to control oily contaminants in the ground at the Rhinehart site. The remedy included implementing a below-ground piping system to collect tainted water, an oil-water separation unit, and a water treatment plant. Wells were drilled to monitor groundwater pollution. The treatment plant eventually handled more than 75 million gal of water contaminated with hydrocarbons and metals.
In September 2000, EPA and the Virginia Department of Environmental Quality (VADEQ) determined that the levels of contaminants in the soil and groundwater in the area had returned to background levels. However, the sediments of the ponds and adjacent stream remained tainted with PAHs, other hydrocarbons, arsenic, cyanide, zinc, and other metals. The final part of the cleanup included removing sediment from the ponds and a section of the Massey Run streambed to a landfill, removing the water treatment plant and its oil-water separator, and revegetating the area.
On Sept. 27, 2002, EPA declared the cleanup complete, and on Sept. 30, 2005, nearly 22 years after the fire began, EPA officially removed the Rhinehart tire fire from the Superfund list. The approximate Superfund cleanup cost: $11.8 million.
The Rhinehart site is still in “postclosing care,” says Graham H. Simmerman Jr., a VADEQ regional land protection manager whose region includes the site. “There’s no longer any monitoring taking place,” Simmerman explains, “but we still make an annual visit.
“Tires remain a major dilemma,” Simmerman says. “Recycling efforts have improved in 30 years, but they still fall well short of the number of tires America generates annually.”
The Rhinehart fire prompted Virginia and most other states to institute waste tire management programs, Simmerman notes. In Virginia, since 1993, some 1,200 tire piles containing nearly 25 million tires have been cleaned up at a cost of $23 million. The cleanup has been promoted through several state initiatives, including a tax on new tires to help pay for the cleanups.
“The worst of the tire piles are gone, but it’s not like they no longer exist,” Simmerman says. With some of the Virginia cleanups, recycling companies come in and shred tires to make rubber chips, he explains. Some landfills have purchased their own shredding equipment. For example, Frederick County uses a mobile shredder that rotates among several landfills in the Shenandoah Valley. Nationally, shredded tires are largely burned to help power cement kilns and paper mills and to generate electricity. Other uses include landfill operations, mulch for playgrounds and athletic fields, as well as fill material for road construction.
The shredded tires in Frederick County end up in the landfill as a lining material, as in Iowa City. “Some people regard using waste material such as shredded tires or ground glass in landfills as recycling,” Simmerman notes. “This is a fine hair to split—it’s still getting buried in the landfill.
“We really need to do something positive with tires that’s not going to cost an arm and a leg for us or future generations,” Simmerman observes. “Converting tires into a liquid fuel like Rhinehart was hoping to do is still a chemical pipe dream for tire recycling, but the value of the oil and air-pollution regulations just doesn’t warrant doing that. I don’t believe Mr. Rhinehart ever made much money on his tires. Maybe he was hopeful that one day it would pay off.”
Getting old tires to pay off in a sustainable way is exactly the goal of organic chemist Alan E. Barton, chief executive officer of Lehigh Technologies, based in Tucker, Ga.
“Chemistry is a part of the fabric of our society, both our infrastructure and our consumer world,” Barton says. “But we are hitting barriers. We are running out of easy sources of reduced carbon, such as petroleum, to use as raw materials. In addition, we are generating mountains of trash, including tire piles. Landfills are becoming difficult to manage. We can’t continue to keep consuming, disposing, and burying.”
This is where Lehigh fits in, Barton explains. “If you make a material once, why can’t you continue to use it over and over again?” Barton and his team started with that concept and developed the idea to turn rubber into a renewable resource.
Tire recyclers get paid for collecting tires, removing metal and fiber, and then processing the rubber into quarter-sized chips. But Lehigh is different, Barton says. “We have taken a specialty chemicals approach to the tire problem to develop a process to commercialize waste rubber as a chemical raw material,” he notes.
The company takes shredded tires from recyclers and waste rubber from rubber-product manufacturers and breaks the material down into micronized rubber powder, or MRP. Through a cryogenic milling process, rubber chips are frozen using liquid nitrogen to make them brittle like glass. The frozen material is then pulverized in a high-speed turbo mill. The pulverized material, ranging from 400-μm granules down to 50-μm powderlike particles, is then warmed up and segregated into size ranges for different applications.
The company’s largest market for MRP is supplementing virgin natural or synthetic rubber in tire manufacturing—more than 200 million tires containing MRP are on the road. The material is also being used in asphalt, driveway sealers, floor tiles, pallets, hoses and gaskets, automotive sound-dampening foams, and plastics.
“The chemistry matters,” Barton says. “Through our R&D we determine how to prepare MRP to meet our customers’ specifications for their products.” Industrial partners that send scrap rubber to Lehigh receive the material back in a form that can be used again as a raw material.
The company began production in 2010 and currently makes about 70 million lb of MRP per year in a 140 million-lb capacity plant. Lehigh sells MRP for roughly half the cost of virgin rubber, Barton notes. In addition, Lehigh’s product requires less energy to produce than virgin rubber, he says, and releases about half as much carbon dioxide as manufacturing new rubber. MRP keeps tires out of landfills altogether.
Barton is convinced that developing infinite cycles of use for rubber and other types of feedstock materials is imperative for chemical firms. “I think this is just the beginning of the next chemical revolution,” he says.
Back in Virginia where the tires burned 30 years ago, the old Rhinehart place is not easy to find. For some passersby, when stopped and asked, the fire is only a fuzzy memory—not like people’s memories of where they were when President Kennedy was shot or when Neil Armstrong took his “one small step for a man” on the moon.
Hiking through the area on a recent rainy day yielded no sign of the conflagration. Deer scampered through grassy fields dotted with pine trees, overlooked by a Cooper’s Hawk sitting in wait on a power line. No tires were in sight.
“I’m glad it’s over,” says Riley, the Frederick County administrator. “Our pain has been the recycling industry’s gain.” As to the future, he hopes the Rhinehart estate will donate the farm to the county so it can be turned into a regional memorial park. “It would be neat if we could pull that off,” Riley says, “to not forget what happened here.”
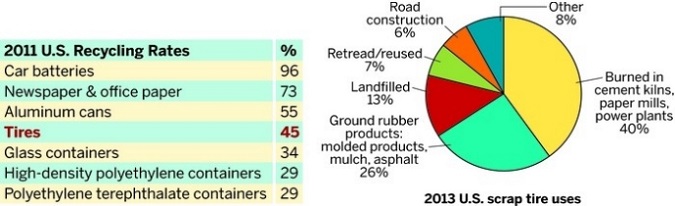
RECYCLING RUBBER
In 2011, the tire recycling rate was average compared with other materials, but the rate is expected to increase as scrap tire uses become more practical and profitable.
It was Paul and Alma Rhinehart’s tire pile. On fire.
Estimates are that the Rhinehart tire-recycling operation had handled as many as 25 million tires in the decade leading up to the fire. The enterprise sold most of the tires for retreading and for ship docking bumpers, floor mats, shoe soles, and other uses. But some 7 million tires that were in too poor condition for resale had accumulated in a pile reaching 80 feet high and strewn across some 5 acres of wooded slopes on the Rhinehart farm. Paul had a plan for these tires—to melt them down to recover and sell crude oil and scrap metal. But the fire got to the tires first.
The Rhinehart tire fire ended up burning for nine months, in the process generating a plume of toxic smoke that spread across four states and a stream of thousands of gallons of crude oil from melting tires that was contained in the nick of time. The environmental disaster created by the tire fire took more than 20 years and nearly $12 million to clean up.
Tire recycling was hindered by a volume problem 30 years ago, and that hasn’t changed. According to the Rubber Manufacturers Association, one waste tire is generated per person per year in the U.S.—today that’s about 315 million tires annually.
But some things have improved. The fire served as a call to action for states to come up with better plans to manage tire recycling. The experience battling the blaze has contributed to advances in environmental monitoring. Cleaning up the fire’s toxic detritus also became one of the few success stories for the Environmental Protection Agency’s Superfund program. But perhaps the fire’s greatest legacy may be that it’s sparking start-up companies to create new technologies for using rubber and other types of feedstock materials as renewable resources.
“The fire was dramatic,” recalls Scott Mason, a photographer for the Winchester Star newspaper. Mason was at home, about 20 miles south of the fire, when he received an urgent call from an editor early that Halloween morning. “Just after dawn, the cloud of smoke looked like the dark outline of just another mountain west of Winchester,” he tells C&EN.
Instead of driving to the fire, Mason drove to the airport, where he found a pilot who was willing to take him up for a look. As he got closer, the mushroom cloud came into dramatic focus, and as Mason started to take photos he saw the wind start catching the smoke and stretching it northward—the smoke drifted for 50 miles across West Virginia and Maryland and into Pennsylvania, and also eastward toward Washington, D.C.
“It was cold up there—below freezing,” Mason continues. “I forgot to bring any gloves, so my hands were getting numb. My eyes were tearing up. I had to duck back inside the airplane window about every two shots. But I got the pictures.” One of his striking shots is on the cover of this issue.
 In 1972, at age 64, Rhinehart had started collecting and recycling tires after Frederick County, Va., banned tires from its landfill. Like many jurisdictions across the U.S., the county faced the problem that whole tires take up too much space in landfills and tend to shift underground over time. Left unburied, though, tires provide a breeding ground for mosquitoes and rats, and they can catch fire.
In 1972, at age 64, Rhinehart had started collecting and recycling tires after Frederick County, Va., banned tires from its landfill. Like many jurisdictions across the U.S., the county faced the problem that whole tires take up too much space in landfills and tend to shift underground over time. Left unburied, though, tires provide a breeding ground for mosquitoes and rats, and they can catch fire.Rhinehart had previously been involved in car salvage operations and conceived of an idea—it’s not clear he ever got it working—to build a disassembly line to melt car parts in increasingly hot temperature zones to recover metals, using propane to burn tires for heat. Tires are made of natural (polyisoprene) or synthetic (styrene-butadiene copolymer) rubber reinforced with metal and/or fiber cords. Burning tires produces about 25% more energy than burning coal. It also produces similar pollutants, and then some.
The car recycling notion seems to have prompted Rhinehart to consider building an incinerator to smelt tires. His thinking was to drop tires into a rotating grate and let heat decompose them. Rhinehart planned to sell the oil and scrap metal and use excess heat from the incinerator to run greenhouses on his farm.
At first, Rhinehart collected tires locally, and then regionally. Eventually he started working up and down the East Coast. Travelers along I-81 through Virginia’s Shenandoah Valley may have seen Rhinehart driving a tractor-trailer truck loaded with tires, with a sign on the door that read: “Old Tire Home, Winchester, Va.”
Rhinehart was part country philosopher, tinkerer, and dreamer—a local legend—recalls Frederick County Administrator John R. Riley Jr., who often found Rhinehart standing in his office. “Mr. Rhinehart definitely had his own idea about what the role of government should be—he would have been a great Tea Party guy, no question about it,” Riley asserts. “He believed in less government, more entrepreneurship, and letting the private sector take the lead.”
But county officials grew weary of asking Rhinehart to stop hoarding the old tires and do something—to get rid of them. After he repeatedly skipped deadlines to quit, Rhinehart lost his charm with county officials. In October 1983, the county filed for a court injunction to cut him off, even as Rhinehart said he was just about ready to crank up his smelter. Days later, on the 31st, came the fire.
That afternoon, Mason, the photographer, drove out to the Rhinehart farm. By then, the place was crawling with firefighters from across the region who had been rousted out of bed to battle the blaze. Heavy flames danced across the top of the glowing tire pile, Mason recounts, but mostly the flames were shrouded by thick black smoke. Mason could see the crews were up against more than they could handle.
Smoke was a foremost concern. Scientists who study tire fires note that the smoke from burning rubber contains noxious sulfur dioxide, deadly carbon monoxide, and cancer-causing polycyclic aromatic hydrocarbons (PAHs), as well as other aromatic and nonaromatic compounds such as styrene and butadiene. “One of our employees and her husband lived in that area, and they have a lake,” Mason remembers. “The soot coming down killed all the fish.”
Behind the smoke screen, a stream of crude oil was forming deep within the tire pile. Like hot lava, the oil started dribbling out, and then later it came in torrents, as much as 200 gal per minute. An oil analysis found it contained benzene, xylenes, anthracene, and other aromatic hydrocarbons, along with some nonaromatic and sulfur compounds. The scientists detected caprolactam, a monomer for nylon fiber used in some tires, and benzothiazole, a degradation product of a compound used as a rubber vulcanizing reagent. The oil analysis also revealed arsenic, cyanide, cadmium, chromium, nickel, zinc, and other metals. The zinc would later become the biggest concern at the Rhinehart fire because its high levels would be toxic to aquatic life.
The greatest threat was the oil reaching Massey Run, a small stream adjacent to the fire, and running into Rhinehart’s farm pond, built by damming the stream. From there, the tarry ooze might reach Hogue Creek half a mile away, and from there the Potomac River 30 miles farther on. It could become a great inland oil spill.
In a Washington Post story, Kory Gabrielsen, a Virginia state hazardous materials officer, summed up the emergency situation this way: “If we don’t stop it … pretty soon it’ll be in Washington and end up in some senator’s martini.”
Firefighters quickly erected filter fences and built a catch basin to trap the oil that hadn’t seeped into the ground. Two weeks later, EPA’s emergency response team constructed a larger concrete-lined containment basin, which was named Dutchman’s Pond, in which to channel the oily waste. Some 840,000 gal of runoff would eventually be contained—most of it was deemed usable and trucked away to be refined into fuel oil. In the end, little of the flowing oil reached Massey Run.
Scientists with Virginia’s Division of Consolidated Laboratory Services analyzed burnt tire by-products in Massey Run and Hogue Creek every two weeks during the fire. The studies appear to be the only published analytical results on the Rhinehart fire.
Using gas chromatography and mass spectrometry, scientists found that only the more water-soluble compounds worked their way downstream. The most common compounds detected included caprolactam and benzothiazole, along with benzoic acid, benzonitrile, and other aromatics. The ensemble of contaminants was sufficiently high in Massey Run to be a concern. But the levels at a site 5 miles farther downstream were low enough so as not to be worrisome.
Scientists today have evolved tools to render a more exact accounting of pollutants in such disasters. Chemistry professor Elizabeth A. Stone of the University of Iowa, for example, studies natural and anthropogenic aerosol particles to assess their impacts on asthma and cardiovascular diseases, as well as on climate. Her team sets up particulate filtering units and then uses solvent extraction followed by gas chromatography and mass spectrometry to detect chemical markers in the aerosols to pinpoint pollution sources and measure pollution levels.
Stone’s group happened to have its monitoring equipment up and running over Memorial Day weekend in 2012 when a tire fire accidentally started in the Iowa City landfill.
One use of old tires today is shredding them to use as a permeable barrier layer to line landfills or as a substitute for soil cover in daily landfill operations. In Iowa City, tires had been shredded into 2- by 2-inch chips and then placed in a 3-foot-thick layer above the wastewater collection system at the bottom of a 7-acre section of the landfill. The fire, which burned an estimated 1.3 million tires and lasted a couple of weeks, produced some 150,000 gal of oil and was put out by covering the shredded tire layer with clay soil. The structural integrity of the landfill contained the Iowa City fire, Stone says, so the oil pollution doesn’t appear to have escaped into the groundwater.
But the smoke was another story. “We were able to use ambient measurements to develop an emission profile for the fire and better understand the chemical composition of the smoke and its potential health effects,” Stone explains. During the course of the fire, her team saw increases in soot (elemental carbon) and PAHs, a sign that the fire transitioned from burning vigorously to smoldering—that is, toward more incomplete combustion.
“PAH levels really shot through the roof,” Stone says. For example, at a site 2 miles from the fire, levels of highly toxic benzo[a]pyrene were up to 30 times as much as the prefire background level.
In addition to Stone’s work at the fire, atmospheric scientist Scott Spak of the University of Iowa’s Public Policy Center performed dispersion modeling, combining emissions estimates from the fire with weather forecasts to predict where the smoke plume would concentrate each day. The information was used to aid firefighting decisions and inform the public. On another front, Iowa chemical engineer Charles O. Stanier used field-deployable instruments on mobile platforms to measure gases such as carbon monoxide and sulfur dioxide and to track the evolution of the shifting smoke plume as it moved farther away from the fire. This type of monitoring wasn’t plausible 30 years ago, Stone says.
“This unexpected incident really sparked a lot of discussion and collaboration between scientists and local government,” Stone says. “It was an interesting opportunity to try to protect human health and learn as much as we can about fires to help other communities respond if something like this happens again.”
At the Rhinehart fire, the firefighting teams did the best they could to control the fire by dousing the tires with water and firefighting foam. They created fire breaks where possible and tried to safely remove tires from the burning pile. Even so, after a couple of weeks the fire still burned and the sky was still smudged with black smoke, according to newspaper reports.
Firefighting experts from around the world were consulted. They offered tried-and-true solutions for putting out oil-well fires, coal-mine fires, and landfill fires. But the interconnected array of air pockets in the haphazard pile of tires made the fire virtually inextinguishable. When a few of the experts came in person to inspect the rubber inferno, they threw their hands up. The situation looked impossible. After several months, EPA officials decided to let the fire determine its own destiny and burn itself out.
On July 4, 1984, it was Independence Day in more ways than one: The Rhinehart fire was declared dead. The pile had turned into a crusty heap prickled with the wiry remains of steel-belted radials.
Nobody seemed to know how the fire got started. Fire officials agreed that igniting the frayed Uniroyals, bald Dunlops, and other tires in the pile took more than a discarded cigarette—it would have taken high heat over an extended period or an open flame for the fire to take hold. Alma Rhinehart, quoted in the Winchester Star the day after the fire started, was certain: “Somebody set it. There’s no question about it.”
In 1987, Melvin Russell Jenkins admitted to torching the tires, although no reason was ever mentioned. Was Rhinehart involved somehow? “Probably not,” Riley says. “He wasn’t like that. Knowing the gentleman as well as I did, that would not have been his intent. He knew what the results would be.” Jenkins later was sentenced to 110 years in prison—less for the Rhinehart fire than for a string of unrelated arsons and a murder.
Rhinehart vowed that the fire wasn’t going to put him out of business. Defying the order to stop storing tires, he brought in more than 1 million more tires. Finally, in February 1991, the Virginia attorney general put a stop to the tire hoarding, citing a public health threat. Paul Rhinehart died, at age 89, on Feb. 23, 1997. Alma Rhinehart died in 2002.
The Rhinehart tire pile was not close to being the world’s largest, and the fire was not the world’s largest tire fire or the one that burned the longest. But the Rhinehart fire was 10 times as large as the biggest previous tire fire in the U.S., and it became part of EPA’s fledgling Superfund program.
Superfund is the common name given to the Comprehensive Environmental Response, Compensation & Liability Act of 1980. This law established a program to remediate an array of hazardous waste sites, including industrial plants, old junkyards, military bases, and gas stations. Superfund was created in the wake of the discovery of several severe cases of toxic waste dumping in the 1970s—Love Canal, in New York, Times Beach, Mo., and the Valley of the Drums, near Louisville, Ky., to name a few.
At the end of 2012, EPA had investigated more than 40,000 potential Superfund sites. The agency has taken action to clean up many of them, but only about 1,680 were bad enough to make it to the official Superfund list. About 370 have been cleaned up and removed from the list, and the Rhinehart tire fire is one of them.
In 1988, EPA instituted a plan to control oily contaminants in the ground at the Rhinehart site. The remedy included implementing a below-ground piping system to collect tainted water, an oil-water separation unit, and a water treatment plant. Wells were drilled to monitor groundwater pollution. The treatment plant eventually handled more than 75 million gal of water contaminated with hydrocarbons and metals.
In September 2000, EPA and the Virginia Department of Environmental Quality (VADEQ) determined that the levels of contaminants in the soil and groundwater in the area had returned to background levels. However, the sediments of the ponds and adjacent stream remained tainted with PAHs, other hydrocarbons, arsenic, cyanide, zinc, and other metals. The final part of the cleanup included removing sediment from the ponds and a section of the Massey Run streambed to a landfill, removing the water treatment plant and its oil-water separator, and revegetating the area.
On Sept. 27, 2002, EPA declared the cleanup complete, and on Sept. 30, 2005, nearly 22 years after the fire began, EPA officially removed the Rhinehart tire fire from the Superfund list. The approximate Superfund cleanup cost: $11.8 million.
The Rhinehart site is still in “postclosing care,” says Graham H. Simmerman Jr., a VADEQ regional land protection manager whose region includes the site. “There’s no longer any monitoring taking place,” Simmerman explains, “but we still make an annual visit.
“Tires remain a major dilemma,” Simmerman says. “Recycling efforts have improved in 30 years, but they still fall well short of the number of tires America generates annually.”
The Rhinehart fire prompted Virginia and most other states to institute waste tire management programs, Simmerman notes. In Virginia, since 1993, some 1,200 tire piles containing nearly 25 million tires have been cleaned up at a cost of $23 million. The cleanup has been promoted through several state initiatives, including a tax on new tires to help pay for the cleanups.
“The worst of the tire piles are gone, but it’s not like they no longer exist,” Simmerman says. With some of the Virginia cleanups, recycling companies come in and shred tires to make rubber chips, he explains. Some landfills have purchased their own shredding equipment. For example, Frederick County uses a mobile shredder that rotates among several landfills in the Shenandoah Valley. Nationally, shredded tires are largely burned to help power cement kilns and paper mills and to generate electricity. Other uses include landfill operations, mulch for playgrounds and athletic fields, as well as fill material for road construction.
The shredded tires in Frederick County end up in the landfill as a lining material, as in Iowa City. “Some people regard using waste material such as shredded tires or ground glass in landfills as recycling,” Simmerman notes. “This is a fine hair to split—it’s still getting buried in the landfill.
“We really need to do something positive with tires that’s not going to cost an arm and a leg for us or future generations,” Simmerman observes. “Converting tires into a liquid fuel like Rhinehart was hoping to do is still a chemical pipe dream for tire recycling, but the value of the oil and air-pollution regulations just doesn’t warrant doing that. I don’t believe Mr. Rhinehart ever made much money on his tires. Maybe he was hopeful that one day it would pay off.”
Getting old tires to pay off in a sustainable way is exactly the goal of organic chemist Alan E. Barton, chief executive officer of Lehigh Technologies, based in Tucker, Ga.
“Chemistry is a part of the fabric of our society, both our infrastructure and our consumer world,” Barton says. “But we are hitting barriers. We are running out of easy sources of reduced carbon, such as petroleum, to use as raw materials. In addition, we are generating mountains of trash, including tire piles. Landfills are becoming difficult to manage. We can’t continue to keep consuming, disposing, and burying.”
This is where Lehigh fits in, Barton explains. “If you make a material once, why can’t you continue to use it over and over again?” Barton and his team started with that concept and developed the idea to turn rubber into a renewable resource.
Tire recyclers get paid for collecting tires, removing metal and fiber, and then processing the rubber into quarter-sized chips. But Lehigh is different, Barton says. “We have taken a specialty chemicals approach to the tire problem to develop a process to commercialize waste rubber as a chemical raw material,” he notes.
The company takes shredded tires from recyclers and waste rubber from rubber-product manufacturers and breaks the material down into micronized rubber powder, or MRP. Through a cryogenic milling process, rubber chips are frozen using liquid nitrogen to make them brittle like glass. The frozen material is then pulverized in a high-speed turbo mill. The pulverized material, ranging from 400-μm granules down to 50-μm powderlike particles, is then warmed up and segregated into size ranges for different applications.
The company’s largest market for MRP is supplementing virgin natural or synthetic rubber in tire manufacturing—more than 200 million tires containing MRP are on the road. The material is also being used in asphalt, driveway sealers, floor tiles, pallets, hoses and gaskets, automotive sound-dampening foams, and plastics.
“The chemistry matters,” Barton says. “Through our R&D we determine how to prepare MRP to meet our customers’ specifications for their products.” Industrial partners that send scrap rubber to Lehigh receive the material back in a form that can be used again as a raw material.
The company began production in 2010 and currently makes about 70 million lb of MRP per year in a 140 million-lb capacity plant. Lehigh sells MRP for roughly half the cost of virgin rubber, Barton notes. In addition, Lehigh’s product requires less energy to produce than virgin rubber, he says, and releases about half as much carbon dioxide as manufacturing new rubber. MRP keeps tires out of landfills altogether.
Barton is convinced that developing infinite cycles of use for rubber and other types of feedstock materials is imperative for chemical firms. “I think this is just the beginning of the next chemical revolution,” he says.
Back in Virginia where the tires burned 30 years ago, the old Rhinehart place is not easy to find. For some passersby, when stopped and asked, the fire is only a fuzzy memory—not like people’s memories of where they were when President Kennedy was shot or when Neil Armstrong took his “one small step for a man” on the moon.
Hiking through the area on a recent rainy day yielded no sign of the conflagration. Deer scampered through grassy fields dotted with pine trees, overlooked by a Cooper’s Hawk sitting in wait on a power line. No tires were in sight.
“I’m glad it’s over,” says Riley, the Frederick County administrator. “Our pain has been the recycling industry’s gain.” As to the future, he hopes the Rhinehart estate will donate the farm to the county so it can be turned into a regional memorial park. “It would be neat if we could pull that off,” Riley says, “to not forget what happened here.”

RECYCLING RUBBER
In 2011, the tire recycling rate was average compared with other materials, but the rate is expected to increase as scrap tire uses become more practical and profitable.
You can return to the main Market News page, or press the Back button on your browser.

