What Does Ocean Acidification Mean for Sea Life?
The sky is low and dusky, and the rain comes in blustery gusts as we make our way out onto a spill of rocks that juts seaward from the shore just north of Boiler Bay on the Oregon coast. Low tide is just beginning; at times it looks as if we’ll be swamped by waves. It’s October 30 and in the late afternoon gloaming, my eyes take a few minutes to adjust so I can begin to differentiate mussels from rock and to spot the clutch of seals watching our progress.
To the scientists who make up the Ocean Margin Ecosystem Group for Acidification Studies, this spot is known as the Fogarty Creek Intertidal Long-Term Ecological Research Site. The obvious drama of this place comes from the waves and wind and charismatic whiskered marine mammals. But I’m here to witness a different kind of drama with Oregon State University graduate student Jeremy Rose, who specializes in marine ecology and is part of a team of scientists investigating the effects of ocean acidification on the small organisms that inhabit the rocky tide-pool landscape beneath our feet.
While it can’t be seen in a glance, what’s happening to the marine environment on the Pacific Northwest coast as a result of the growing concentration of carbon dioxide in Earth’s atmosphere is indeed dramatic. Since the mid-18th century, human activity—mainly fossil fuel burning—has increased the atmospheric concentration of CO2 by about 40 percent. Because oceans absorb about a quarter of the CO2 released into the atmosphere each year, as more CO2 enters the atmosphere, more ends up in the ocean. “Think of carbon as a global pollutant that affects the ocean everywhere it touches the sky,” explains Stanford University marine science professor and Hopkins Marine Station director Steve Palumbi.
As CO2 dissolves in seawater, chemical reactions produce an acid. Over the past 250 or so years, the acidity of the world’s oceans has increased 30 percent. Scientists believe oceans have not experienced the current level of acidity in about 2 million years. Not only that, but according to National Oceanic and Atmospheric Administration senior scientist Richard Feely, conditions are changing faster than anything seen in geologic history. If today’s global CO2 emission trends continue, scientists estimate that by the end of this century, oceans will be more acidic than they have been for more than 20 million years.
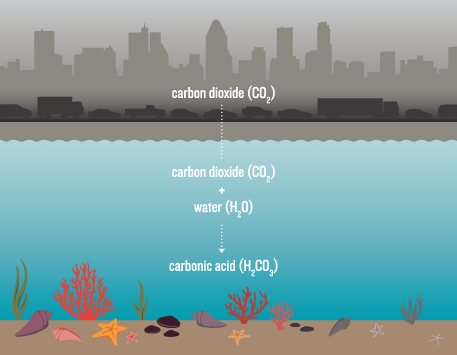
Carbon dioxide given off by vehicles, power plants and other human sources spells trouble for many marine organisms. The gas combines with seawater to form carbonic acid, which reduces availability of the carbonate ions they need to build shells and other structures. Acidification also appears to disrupt physiological processes.
And that’s a problem. The rise in dissolved CO2 and concurrent drop in pH (lower pH indicates higher acidity), changes ocean chemistry in a way that robs marine organisms, such as mollusks and corals, of the carbonate ions they need to build shells and skeletons. At the same time, the increasing acidity can erode the structures they’ve already built, and appears capable of disrupting their bodies in other ways that make it hard for them to thrive. This is bad news not only for the organisms themselves, but also for people who rely on them for food and jobs, and perhaps even more profoundly, for the stability of the ecosystems with which they—and we—are intertwined.
Investigating Impacts
The chemistry behind ocean acidification is well understood. What scientists are working on now is trying to understand what is happening within marine organisms and their coastal communities as the ocean’s pH drops at the same time marine environments experience other stressors such as warming temperatures, pollution and overfishing.
Among their big questions: Can marine species adapt to this rapid change, and if so, how? Or as Morgan Kelly, a postdoctoral researcher studying ocean acidification impacts at the University of California, Santa Barbara, puts it, “Will evolution come to the rescue?”
To begin to answer this question, scientists are exploring how—down to the subcellular level—marine species are responding biologically to acidification. They are also examining how individual species’ responses may affect marine ecosystems. An adverse impact to one species, or conditions that overwhelmingly favor another, can create imbalances in the marine food web and lead to survival problems for a whole suite of species. And on an even larger scale, scientists are investigating what such changes may mean for fisheries and the people who depend on them, and how marine policy and conservation might respond.
Laboratory experiments are part of the picture. But because ocean conditions are so complex and difficult to replicate, scientists are also conducting research in places like Fogarty Creek. The OMEGAS project, which includes study sites along the northern California and Oregon coast, is tracking ocean pH with offshore sensors while monitoring what’s happening biologically at these sites to intertidal species as seawater becomes more acidic. As UC Santa Barbara professor Gretchen Hofmann explained at the 2012 Ocean in a High CO2 World meeting held in Monterey, Calif., in September, scientists are investigating the “fine tuning of populations to their local environment” in locations now experiencing the most dramatically lowered pH.
Purple Urchin
As Rose and I walk out on the rocks, at first I see only boulders and water. But as I crouch to get a better look, an intricate world comes into focus. Yards of pearly black mussels are punctuated by patches of pale whorled pointy shells of gooseneck barnacles. Beneath the surface of the water, trapped in small pools as the tide recedes, are clusters of anemones that look like upside-down branchless coral. I spot a few fat pink sea stars and several distinct types of algae. Among these are long, bright-green rubbery streamers, short dull olive bristly algae and delicate lacy salmon-colored coralline algae, named for the calcareous skeleton that looks like bones of an exceptionally tiny bird. Deeper underwater, nestled among the anemones, are the creatures we have come to see: Strongylocentrotus purpuratus, the purple sea urchin.
Purple sea urchins are of interest to marine biologists studying ocean acidification for numerous reasons. These creatures live up and down the Pacific Coast where pH is changing markedly. Their habitat is one that naturally varies greatly with the ebb and flow of tides. It is also a highly structured community of species in which the sea urchins play an important role, as a food source for sea otters, in controlling algae and as a component of a healthy ecosystem. They’re also a well-studied species—so well studied that their entire genome has been sequenced, enabling scientists to investigate genetic impacts of ocean acidification. This information is essential to understanding the species’ future and how their fate may affect other ecological community members.
Because the pH recorded on the Oregon coast is much lower than that in California (thanks to ocean circulation, seasonal winds and upwelling), how the northerly purple sea urchins are responding to ocean acidification will help scientists understand what may happen to this whole community of species as ocean pH drops further, explains Kelly. It appears that seawater pH affects how hard the urchins must work to maintain the biochemical balance within their cells. That some seem to be “doing okay” under lower pH doesn’t mean that all is well, says Kelly. It means they’re doing something to compensate.
Tyler Evans, Kelly’s colleague at UC Santa Barbara, is an environmental physiologist and postdoctoral fellow investigating how higher dissolved CO2 and lower pH affect sea urchins’ genes. By looking at the individual genes, he hopes to see exactly which are being altered by the changes in seawater chemistry and how ocean acidification is affecting the genes’ ability to make proteins—among the most basic building blocks of life. Thus far, Evans explains, they’ve identified important changes in how sea urchins’ cells transport calcium and sodium. A balance of these is vital to urchins, both for maintaining healthy cell function and for shell building. If sea urchins have to work harder to maintain this balance, it could affect their development or ability to reproduce. If urchins fail to thrive, it would likely have an adverse affect on their entire community of mussels, sea stars, anemones, fish and marine mammals.
“The behavioral and energy changes needed to maintain yourself as a species are really complicated,” explains National Center for Atmospheric Research scientist Joanie Kleypas, a pioneering ocean acidification researcher. Not only that, notes Stanford’s Palumbi, the costs of coping with changes such ocean acidification may take more than one generation to become apparent.
Similar effects have been observed for species other than the purple sea urchin. In lab experiments, green sea urchin larvae exposed to low pH have grown more slowly and developed physiological abnormalities. Mussels exposed experimentally to low pH appeared to have increased metabolic rates, reduced reproduction and some immune system suppression—all clear indications that acidified conditions are adversely affecting these animals’ physiological functions.
Natural Laboratories
To investigate the ecological impacts of acidification over the long term, scientists are also studying what are effectively natural laboratories for high CO2—places where the gas bubbles up through vents in the ocean floor. One such site is in the Mediterranean, where UC Davis Bodega Marine Lab postdoctoral researcher Kristy Kroeker and colleagues are studying how these conditions affect the ecology of the local reef community.
Reef communities are typically very biologically diverse, with numerous species that each play important roles in the community’s physical structure and food web, explains Kroeker. But under high CO2 conditions, certain algae begin to dominate while the coralline algae that depend on calcium carbonate fare less well, changing what the community provides in the way of food and shelter. Kroeker and her colleagues are investigating how seemingly small changes in these food and structure roles will play out on an ecosystem scale and how this compares to acidification-related changes at sites like Fogarty Creek.
As Kleypas explains, such studies will help us understand if “a community is going to change a lot or not” under ocean acidification and how any changes that do occur might affect the community’s ecological resilience. Changes to a community’s anchor or keystone species—one that plays a crucial role in an ecosystem’s function—she explains, are the most likely to affect the whole food web.
By learning which species are most vulnerable to acidification and which are better able to adapt, scientists can target conservation measures aimed at protecting those species, explains Kelly. This could involve curtailing other pollutants or development that’s adversely impacting vulnerable species and habitat, including by identifying potential reserves. Such actions can’t remove excess CO2 already in the system, but they can help build resilience. This could be particularly helpful where important fisheries may be affected, says Kelly. But, cautions Evans, “we really don’t know yet what it takes to survive in a low pH ocean, and we need that information to set conservation priorities.”
Get a Grip
Yet these are but short-term strategies as the world tries to get a grip on the carbon emissions that are ultimately responsible for ocean acidification.
“First and foremost,” says NOAA outgoing administrator Jane Lubchenco, “we need to demand that our elected representatives take seriously the need to reduce carbon emissions, and that’s true at a national level but also at the local level.”
The process of ocean acidification, like the other manifestations of climate change prompted by excessive atmospheric CO2, now cannot be reversed entirely. But with swift and dramatic action, the rate of change might be slowed. And to help lessen acidification’s impacts, scientists suggest addressing not only carbon emissions but other environmental stressors that can exacerbate these effects as well. We “also need to reduce other sources of pollution,” including excess nutrients from both urban and rural sources, Lubchenco says.
This is exactly the strategy Washington state’s Blue Ribbon Panel on Ocean Acidification recommended in a report released in November. The report formed the basis of an executive order signed by Washington governor Chris Gregoire the same day. The first such policy aimed at tackling ocean acidification, both the report and the executive order (designed to implement the report’s recommendations), combine strategies to reduce CO2 emissions and other pollution that exacerbates acidification, along with $3.3 million in funding for research and implementation. The recommendations are also be part of legislation Washington state senator and blue ribbon panel member Kevin Ranker recently introduced—and that he says he hopes will be copied by other coastal states.
“We have a lot of work to do,” says Lubchenco, noting that most people in the U.S. have not yet heard of ocean acidification. “But,” she says, “if they like eating oysters or salmon or enjoy watching whales or scuba diving in coral reefs, they should be paying attention—because it’s a serious threat.”
“The basic policy message,” says Palumbi, is that carbon emissions are “a global pollutant, and we have to fix this problem.” While a shellfish hatchery may be able to control the chemistry of water in its tanks or choose a different species to farm, the same can’t be done in the world’s wild oceans.
In the meantime, as effects of ocean acidification play out, scientists and policy makers continue the quest to understand how individual species and marine communities will fare and how this information can be used to protect them before even more dramatic changes occur. “When it comes to ocean acidification,” says Lubchenco, “we’re all still explorers.”
To the scientists who make up the Ocean Margin Ecosystem Group for Acidification Studies, this spot is known as the Fogarty Creek Intertidal Long-Term Ecological Research Site. The obvious drama of this place comes from the waves and wind and charismatic whiskered marine mammals. But I’m here to witness a different kind of drama with Oregon State University graduate student Jeremy Rose, who specializes in marine ecology and is part of a team of scientists investigating the effects of ocean acidification on the small organisms that inhabit the rocky tide-pool landscape beneath our feet.
While it can’t be seen in a glance, what’s happening to the marine environment on the Pacific Northwest coast as a result of the growing concentration of carbon dioxide in Earth’s atmosphere is indeed dramatic. Since the mid-18th century, human activity—mainly fossil fuel burning—has increased the atmospheric concentration of CO2 by about 40 percent. Because oceans absorb about a quarter of the CO2 released into the atmosphere each year, as more CO2 enters the atmosphere, more ends up in the ocean. “Think of carbon as a global pollutant that affects the ocean everywhere it touches the sky,” explains Stanford University marine science professor and Hopkins Marine Station director Steve Palumbi.
As CO2 dissolves in seawater, chemical reactions produce an acid. Over the past 250 or so years, the acidity of the world’s oceans has increased 30 percent. Scientists believe oceans have not experienced the current level of acidity in about 2 million years. Not only that, but according to National Oceanic and Atmospheric Administration senior scientist Richard Feely, conditions are changing faster than anything seen in geologic history. If today’s global CO2 emission trends continue, scientists estimate that by the end of this century, oceans will be more acidic than they have been for more than 20 million years.

Carbon dioxide given off by vehicles, power plants and other human sources spells trouble for many marine organisms. The gas combines with seawater to form carbonic acid, which reduces availability of the carbonate ions they need to build shells and other structures. Acidification also appears to disrupt physiological processes.
And that’s a problem. The rise in dissolved CO2 and concurrent drop in pH (lower pH indicates higher acidity), changes ocean chemistry in a way that robs marine organisms, such as mollusks and corals, of the carbonate ions they need to build shells and skeletons. At the same time, the increasing acidity can erode the structures they’ve already built, and appears capable of disrupting their bodies in other ways that make it hard for them to thrive. This is bad news not only for the organisms themselves, but also for people who rely on them for food and jobs, and perhaps even more profoundly, for the stability of the ecosystems with which they—and we—are intertwined.
Investigating Impacts
The chemistry behind ocean acidification is well understood. What scientists are working on now is trying to understand what is happening within marine organisms and their coastal communities as the ocean’s pH drops at the same time marine environments experience other stressors such as warming temperatures, pollution and overfishing.
Among their big questions: Can marine species adapt to this rapid change, and if so, how? Or as Morgan Kelly, a postdoctoral researcher studying ocean acidification impacts at the University of California, Santa Barbara, puts it, “Will evolution come to the rescue?”
To begin to answer this question, scientists are exploring how—down to the subcellular level—marine species are responding biologically to acidification. They are also examining how individual species’ responses may affect marine ecosystems. An adverse impact to one species, or conditions that overwhelmingly favor another, can create imbalances in the marine food web and lead to survival problems for a whole suite of species. And on an even larger scale, scientists are investigating what such changes may mean for fisheries and the people who depend on them, and how marine policy and conservation might respond.
Laboratory experiments are part of the picture. But because ocean conditions are so complex and difficult to replicate, scientists are also conducting research in places like Fogarty Creek. The OMEGAS project, which includes study sites along the northern California and Oregon coast, is tracking ocean pH with offshore sensors while monitoring what’s happening biologically at these sites to intertidal species as seawater becomes more acidic. As UC Santa Barbara professor Gretchen Hofmann explained at the 2012 Ocean in a High CO2 World meeting held in Monterey, Calif., in September, scientists are investigating the “fine tuning of populations to their local environment” in locations now experiencing the most dramatically lowered pH.
Purple Urchin
As Rose and I walk out on the rocks, at first I see only boulders and water. But as I crouch to get a better look, an intricate world comes into focus. Yards of pearly black mussels are punctuated by patches of pale whorled pointy shells of gooseneck barnacles. Beneath the surface of the water, trapped in small pools as the tide recedes, are clusters of anemones that look like upside-down branchless coral. I spot a few fat pink sea stars and several distinct types of algae. Among these are long, bright-green rubbery streamers, short dull olive bristly algae and delicate lacy salmon-colored coralline algae, named for the calcareous skeleton that looks like bones of an exceptionally tiny bird. Deeper underwater, nestled among the anemones, are the creatures we have come to see: Strongylocentrotus purpuratus, the purple sea urchin.
Purple sea urchins are of interest to marine biologists studying ocean acidification for numerous reasons. These creatures live up and down the Pacific Coast where pH is changing markedly. Their habitat is one that naturally varies greatly with the ebb and flow of tides. It is also a highly structured community of species in which the sea urchins play an important role, as a food source for sea otters, in controlling algae and as a component of a healthy ecosystem. They’re also a well-studied species—so well studied that their entire genome has been sequenced, enabling scientists to investigate genetic impacts of ocean acidification. This information is essential to understanding the species’ future and how their fate may affect other ecological community members.
Because the pH recorded on the Oregon coast is much lower than that in California (thanks to ocean circulation, seasonal winds and upwelling), how the northerly purple sea urchins are responding to ocean acidification will help scientists understand what may happen to this whole community of species as ocean pH drops further, explains Kelly. It appears that seawater pH affects how hard the urchins must work to maintain the biochemical balance within their cells. That some seem to be “doing okay” under lower pH doesn’t mean that all is well, says Kelly. It means they’re doing something to compensate.
Tyler Evans, Kelly’s colleague at UC Santa Barbara, is an environmental physiologist and postdoctoral fellow investigating how higher dissolved CO2 and lower pH affect sea urchins’ genes. By looking at the individual genes, he hopes to see exactly which are being altered by the changes in seawater chemistry and how ocean acidification is affecting the genes’ ability to make proteins—among the most basic building blocks of life. Thus far, Evans explains, they’ve identified important changes in how sea urchins’ cells transport calcium and sodium. A balance of these is vital to urchins, both for maintaining healthy cell function and for shell building. If sea urchins have to work harder to maintain this balance, it could affect their development or ability to reproduce. If urchins fail to thrive, it would likely have an adverse affect on their entire community of mussels, sea stars, anemones, fish and marine mammals.
“The behavioral and energy changes needed to maintain yourself as a species are really complicated,” explains National Center for Atmospheric Research scientist Joanie Kleypas, a pioneering ocean acidification researcher. Not only that, notes Stanford’s Palumbi, the costs of coping with changes such ocean acidification may take more than one generation to become apparent.
Similar effects have been observed for species other than the purple sea urchin. In lab experiments, green sea urchin larvae exposed to low pH have grown more slowly and developed physiological abnormalities. Mussels exposed experimentally to low pH appeared to have increased metabolic rates, reduced reproduction and some immune system suppression—all clear indications that acidified conditions are adversely affecting these animals’ physiological functions.
Natural Laboratories
To investigate the ecological impacts of acidification over the long term, scientists are also studying what are effectively natural laboratories for high CO2—places where the gas bubbles up through vents in the ocean floor. One such site is in the Mediterranean, where UC Davis Bodega Marine Lab postdoctoral researcher Kristy Kroeker and colleagues are studying how these conditions affect the ecology of the local reef community.
Reef communities are typically very biologically diverse, with numerous species that each play important roles in the community’s physical structure and food web, explains Kroeker. But under high CO2 conditions, certain algae begin to dominate while the coralline algae that depend on calcium carbonate fare less well, changing what the community provides in the way of food and shelter. Kroeker and her colleagues are investigating how seemingly small changes in these food and structure roles will play out on an ecosystem scale and how this compares to acidification-related changes at sites like Fogarty Creek.
As Kleypas explains, such studies will help us understand if “a community is going to change a lot or not” under ocean acidification and how any changes that do occur might affect the community’s ecological resilience. Changes to a community’s anchor or keystone species—one that plays a crucial role in an ecosystem’s function—she explains, are the most likely to affect the whole food web.
By learning which species are most vulnerable to acidification and which are better able to adapt, scientists can target conservation measures aimed at protecting those species, explains Kelly. This could involve curtailing other pollutants or development that’s adversely impacting vulnerable species and habitat, including by identifying potential reserves. Such actions can’t remove excess CO2 already in the system, but they can help build resilience. This could be particularly helpful where important fisheries may be affected, says Kelly. But, cautions Evans, “we really don’t know yet what it takes to survive in a low pH ocean, and we need that information to set conservation priorities.”
Get a Grip
Yet these are but short-term strategies as the world tries to get a grip on the carbon emissions that are ultimately responsible for ocean acidification.
“First and foremost,” says NOAA outgoing administrator Jane Lubchenco, “we need to demand that our elected representatives take seriously the need to reduce carbon emissions, and that’s true at a national level but also at the local level.”
The process of ocean acidification, like the other manifestations of climate change prompted by excessive atmospheric CO2, now cannot be reversed entirely. But with swift and dramatic action, the rate of change might be slowed. And to help lessen acidification’s impacts, scientists suggest addressing not only carbon emissions but other environmental stressors that can exacerbate these effects as well. We “also need to reduce other sources of pollution,” including excess nutrients from both urban and rural sources, Lubchenco says.
This is exactly the strategy Washington state’s Blue Ribbon Panel on Ocean Acidification recommended in a report released in November. The report formed the basis of an executive order signed by Washington governor Chris Gregoire the same day. The first such policy aimed at tackling ocean acidification, both the report and the executive order (designed to implement the report’s recommendations), combine strategies to reduce CO2 emissions and other pollution that exacerbates acidification, along with $3.3 million in funding for research and implementation. The recommendations are also be part of legislation Washington state senator and blue ribbon panel member Kevin Ranker recently introduced—and that he says he hopes will be copied by other coastal states.
“We have a lot of work to do,” says Lubchenco, noting that most people in the U.S. have not yet heard of ocean acidification. “But,” she says, “if they like eating oysters or salmon or enjoy watching whales or scuba diving in coral reefs, they should be paying attention—because it’s a serious threat.”
“The basic policy message,” says Palumbi, is that carbon emissions are “a global pollutant, and we have to fix this problem.” While a shellfish hatchery may be able to control the chemistry of water in its tanks or choose a different species to farm, the same can’t be done in the world’s wild oceans.
In the meantime, as effects of ocean acidification play out, scientists and policy makers continue the quest to understand how individual species and marine communities will fare and how this information can be used to protect them before even more dramatic changes occur. “When it comes to ocean acidification,” says Lubchenco, “we’re all still explorers.”
You can return to the main Market News page, or press the Back button on your browser.

