The rechargeable revolution: A better battery
The mobile world depends on lithium-ion batteries — today’s ultimate rechargeable energy store. Last year, consumers bought five billion Li-ion cells to supply power-hungry laptops, cameras, mobile phones and electric cars. “It is the best battery technology anyone has ever seen,” says George Crabtree, director of the US Joint Center for Energy Storage Research (JCESR), which is based at the Argonne National Laboratory near Chicago, Illinois. But Crabtree wants to do much, much better.
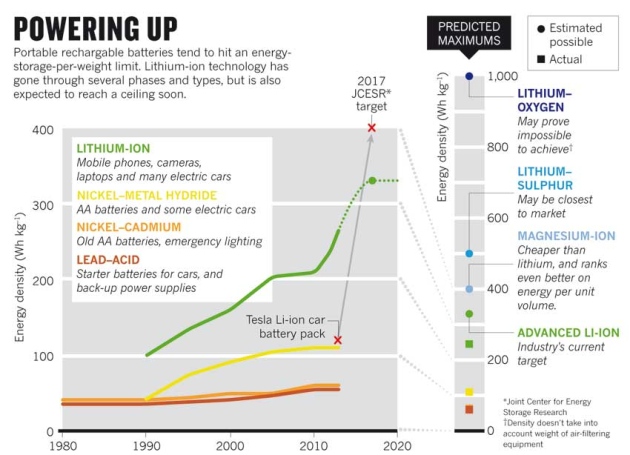
Modern Li-ion batteries hold more than twice as much energy by weight as the first commercial versions sold by Sony in 1991 — and are ten times cheaper. But they are nearing their limit. Most researchers think that improvements to Li-ion cells can squeeze in at most 30% more energy by weight. That means that Li-ion cells will never give electric cars the 800-kilometre range of a petrol tank, or supply power-hungry smartphones with many days of juice.
In 2012, the JCESR hub won US$120 million from the US Department of Energy to take a leap beyond Li-ion technology. Its stated goal was to make cells that, when scaled up to the sort of commercial battery packs used in electric cars, would be five times more energy dense than the standard of the day, and five times cheaper, in just five years. That means hitting a target of 400 watt-hours per kilogram (Wh kg1) by 2017.
Crabtree calls the goal “very aggressive”; veteran battery researcher Jeff Dahn at Dalhousie University in Halifax, Canada, calls it “impossible”. The energy density of rechargeable batteries has risen only sixfold since the early lead–nickel rechargeables of the 1900s. But, says Dahn, the JCESR’s target focuses attention on technologies that will be crucial in helping the world to switch to renewable energy sources — storing up solar energy for night-time or a rainy day, for example. And the US hub is far from alone. Many research teams and companies in Asia, the Americas and Europe are looking beyond Li-ion, and are pursuing strategies that may topple it from its throne.
Lose the dead weight
Chemical engineer Elton Cairns suspected he had tamed a promising-but-wild battery chemistry early last year, when his coin-sized cells were still going strong even after a few months of continual draining and recharging. By July, his cells at the Lawrence Berkeley National Laboratory in Berkeley, California, had cycled 1,500 times and had lost only half of their capacity1 — a performance roughly on a par with the best Li-ion batteries.
His batteries are based on lithium–sulphur (Li–S) technology, which uses extremely cheap materials and in theory can pack in five times more energy by weight than Li-ion (in practice, researchers suspect, it will probably be only twice as much). Li–S batteries were first posited 40 years ago, but researchers could not get them to survive past about 100 cycles. Now, many think that the devices are the technology closest to becoming a commercially viable successor to Li-ion.
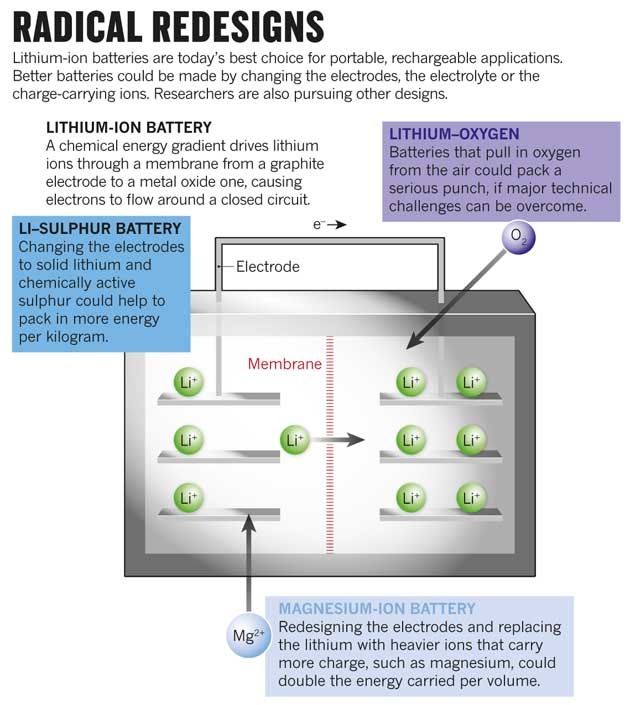
One of Li–S’s main advantages, says Cairns, is that it gets rid of the “dead weight” in a Li-ion battery. Inside a typical Li-ion cell, space is taken up by a layered graphite electrode that does little more than host lithium ions. These ions flow through a charge-carrying liquid electrolyte into a layered metal oxide electrode. As with all batteries, current is generated because electrons must flow around an outside circuit to balance the charges. To recharge the battery, a voltage is applied to reverse the electron flow, which also drives the lithium ions back.
In a Li–S battery, the graphite is replaced by a sliver of pure lithium metal that does double duty as both the electrode and the supplier of lithium ions: it shrinks as the battery runs, and reforms when the battery is recharged. And the metal oxide is replaced by cheaper, lighter sulphur that can really pack the lithium in: each sulphur atom bonds to two lithium atoms, whereas it takes more than one metal atom to bond to just one lithium. All of that creates a distinct weight and cost advantage for Li–S technology.
But the reaction between lithium and sulphur causes a problem. As the battery is charged and discharged, soluble Li–S compounds can seep into the electrolyte, degrading the electrodes so that the battery loses charge and the cell gums up. To prevent this, Cairns uses tricks made possible by advances in nanotechnology and electrolyte chemistry — including adulterating his sulphur electrode with graphene oxide binders, and using specially designed electrolytes that do not dissolve lithium and sulphur so much. Cairns predicts that a commercial-sized cell could achieve an energy-density of around 500 Wh kg1. Other labs are reporting similar results, he says.
Some researchers doubt that the academic cheer will translate into commercial success. Laboratories often use low proportions of sulphur and lots of electrolyte, which is relatively easy to work with but does not create an energy-dense battery. Bumping up the sulphur and decreasing the electrolyte makes the cell more likely to gum up, says Steve Visco, who has spent more than 20 years working on Li–S at battery firm PolyPlus in Berkeley, just 5 kilometres west of Cairns’ lab. Making a cheap commercial cell that works over a range of temperatures will also be hard, he says.
At least one company stands by Li–S’s prospects: Oxis Energy in Abingdon, UK. It says it has run large cells for an impressive 900 cycles, at energy densities that match current Li-ion cells. Oxis is working with Lotus Engineering, headquartered in Ann Arbor, Michigan, on a project to reach 400 Wh kg1 by 2016 for an electric vehicle.
Pack more punch per ion
As the world’s lightest metal, lithium provides a huge weight advantage. But some researchers argue that the next generation of cells should switch to heavier elements such as magnesium. Unlike lithium ions, which can carry only one electrical charge each, doubly charged magnesium ions shuttle two at a time — instantly multiplying the electrical energy that can be released for the same volume.
Magnesium comes with its own challenge, however: whereas lithium zips through electrolytes and electrodes, magnesium with its two charges moves as if through treacle.
Peter Chupas, a battery researcher at Argonne National Laboratory who is working with the JCESR, is shooting high-energy X-rays at magnesium in various electrolytes to investigate why it experiences so much drag. So far, he and his colleagues have found that magnesium exerts a strong pull on oxygen atoms in any surrounding solvent, attracting clusters of solvent molecules that make it bulkier. That kind of basic research is key to creating a better battery, but it is not usually done by industry, says Crabtree. “The typical R&D operation operates on trial and error, not fundamental research,” he says. This, he says, is where JCESR is bringing an advantage to the field.
Materials scientist Kristin Persson at Lawrence Berkeley is using a supercomputer to simulate the innards of possible new batteries, trying to find a combination of electrodes and electrolytes that will allow magnesium to pass through more easily. “Right now, we are crunching through around 2,000 different electrolytes,” she says.
Persson and Gerbrand Ceder, a materials scientist at the Massachusetts Institute of Technology in Cambridge, founded a company to develop these higher-charge-carrying batteries. Pellion Technologies, based in Cambridge, is tight-lipped about its results; it has published only one paper about electrolytes2. A spate of patents published in late 2013 hint that the company is developing more-open electrode structures to help the magnesium ions to flow. Major electronics firms such as Toyota, LG, Samsung and Hitachi are also working on such cells, releasing little information beyond occasional teasers.
As companies jostle in secret, Persson continues to run through what she calls the “electrolyte genome”. The sifting-by-supercomputer approach could also help the search for batteries made with other multiple-charge-carrying (or ‘multivalent’) metals, such as aluminium and calcium. Ceder urges patience, pointing out that research into Li-ion battery chemistry has enjoyed a 40-year head start. “We have so little information about multivalent ions,” he says.
Make batteries that breathe
Winfried Wilcke, who describes himself as “an extremely happy owner of a Tesla S” electric car, credits the vehicle with changing his mind about battery-research priorities.
Five years ago, Wilcke, who heads IBM’s nanoscience and technology division in San Jose, California, launched a project to develop a car battery with an 800-kilometre range. At the start, he focused on the theoretical ultimate in energy-dense electrochemical storage: the oxidation of lithium with oxygen drawn from the air. Such ‘breathing’ batteries have a huge weight advantage over other types, because they do not have to carry around one of their main ingredients. A lithium–oxygen (Li–O) battery can, in theory, store energy as densely as a petrol engine — more than ten times better than today’s car battery packs.
But after driving more than 22,000 kilometres in his electric roadster, Wilcke is happy with the 400-kilometre range that its battery already provides. The real problem, he says, is money: battery packs for electric cars cost more than $500 kWh1. “What’s holding back the mass acceptance of electric cars is really the price rather than the energy density,” he says. So Wilcke now favours a cheaper breathing battery based on sodium. Theory predicts that sodium–oxygen (Na–O) batteries could provide only half the energy density of Li–O, but that is still five times better than Li-ion batteries. And sodium is cheaper than lithium, so Na–O might, Wilcke hopes, get closer to the $100-kWh1 goal that the JCESR and others have set for affordability.
Wilcke’s change of heart was undoubtedly influenced by the fact that many have given up hope on Li–O. Researchers who have tried to make it work over the past 20 years have wrestled with unwanted side reactions: carbon in the electrolyte and electrode material react with the lithium and oxygen to form lithium carbonate, so that in every cycle, some 5–10% of the battery capacity is lost. After 50 cycles or so, the battery suffocates. “The bottom line is that Li–O has zero chance for vehicles,” says Stanley Whittingham at Binghamton University in New York, who invented the concept of Li-ion batteries in the 1970s and still focuses on squeezing the best performance out of them. Researchers hoping to resuscitate Li–O include Peter Bruce, a chemist at the University of St Andrews, UK. “We are closer to what’s needed than we were a few years ago,” he argues. But many consider it a lost cause.
Wilcke took an interest in the sodium breathing battery last year, following a surprising discovery by a team including Jürgen Janek and Philipp Adelhelm at the Justus-Liebig University of Giessen in Germany. They found that a Na–O battery recharges more efficiently than Li–O, without complicating side-reactions3. “We tried it and were pretty stunned,” says Wilcke. Plus, he says, it works with cheap electrodes and electrolytes. Janek says that his team has now shown that its battery can work reversibly for at least 100 cycles — not bad for the early days of the technology. Chemicals giant BASF is now working with them.
Dahn, for one, is not convinced. Debate rages about whether breathing batteries will require heavy filtering equipment to extract oxygen from the air, which would cut down or even eliminate their energy-per-weight advantage. “Na–O is just the latest craze,” says Dahn. But Wilcke is willing to bet otherwise.
Go big for the grid
Donald Sadoway’s vision of the future battery looks like a smelting plant: he envisions crates the size of shipping containers, each holding 20 refrigerator-sized steel blocks containing litres of molten metals and salts heated to 500 °C.
Such batteries could never fit in a car, and cannot beat Li-ion on measures such as energy stored per unit weight. But when it comes to storing energy for the electricity grid — or other non-portable applications — size does not matter. Instead of a small, light battery that packs a powerful punch, what people need is a battery that cheaply bottles and releases small-to-large amounts of electricity without much maintenance. The JCESR wants such batteries to last for 7,000 cycles, or about 20 years.
“The field is wide open,” says Ceder. Grid suppliers have used banks of cheap, old-fashioned lead–acid batteries, for example, or stacks of Li-ion. A dizzying array of other chemistries are in development, including zinc–air and sodium-ion. Most technologies are doing well to cost five times as much as the JCESR’s $100-kWh1 target.
Sadoway, a materials chemist at the Massachusetts Institute of Technology, is developing an alternative with two layers of molten metal as electrodes, separated by their different densities and by a layer of molten-salt electrolyte. The metal layers swell or shrink as ions pass between them, storing or releasing energy. Because everything is liquid, there is nothing that could crack after thousands of cycles, as solid electrodes might.
Crabtree, Dahn and other researchers worry about the energy needed to keep the components molten. But Sadoway says that the charging and discharging processes produce enough heat on their own. His company — Ambri in Marlborough, Massachusetts — plans to install test batteries in Hawaii and at a military base on Cape Cod, Massachusetts, this year, each supplying tens of kilowatt-hours.
Other research groups are pursuing less-radical flow batteries, in which the fuel consists of two liquids that pass ions to each other through a membrane. The liquids can be kept in tanks outside the battery and pumped in to flow past each other when needed, so it is possible to store larger amounts of energy indefinitely simply by using bigger tanks. But they do need pumps and valves, which Sadoway says will require maintenance.
Commercial flow batteries use vanadium ions in the liquid on both sides of the barrier. But vanadium and the membranes are expensive: the world’s largest flow battery, installed at a wind farm in China, probably costs $1,000 kWh1, estimates Huamin Zhang at the Chinese Academy of Sciences’ Dalian Institute of Chemical Physics. “The cost of vanadium just kills you,” says Michael Aziz, a materials scientist at Harvard University in Cambridge, Massachusetts.
In January this year, a team including Aziz announced4 that cheap organic chemicals called quinones could be used in a flow battery, partnered to a standard liquid electrode such as bromine. Aziz has cycled his system more than 100 times and it is still running strong. He hopes that he can get such batteries below the magic $100 kWh1, but “this is a toy in a fume hood in a laboratory right now”, he says. “There is no way to know the true cost until you are mass-producing it.”
Crabtree calls the work “promising” and says that the JCESR is also looking at organic chemicals for flow batteries. Another option it is pursuing is to use liquid Li–S and solid lithium in a sort of half-flow battery.
“It’s early days: people are looking at really oddball systems, and everyone’s trying to figure out how to get the lifetime up and the costs down,” says Dahn. The JCESR, for one, is hoping that basic research can fill in the gaps and make these technologies work. “The beyond-lithium-ion space is rich with opportunity,” says Crabtree, “and mostly unexplored.”
Modern Li-ion batteries hold more than twice as much energy by weight as the first commercial versions sold by Sony in 1991 — and are ten times cheaper. But they are nearing their limit. Most researchers think that improvements to Li-ion cells can squeeze in at most 30% more energy by weight. That means that Li-ion cells will never give electric cars the 800-kilometre range of a petrol tank, or supply power-hungry smartphones with many days of juice.
In 2012, the JCESR hub won US$120 million from the US Department of Energy to take a leap beyond Li-ion technology. Its stated goal was to make cells that, when scaled up to the sort of commercial battery packs used in electric cars, would be five times more energy dense than the standard of the day, and five times cheaper, in just five years. That means hitting a target of 400 watt-hours per kilogram (Wh kg1) by 2017.
Crabtree calls the goal “very aggressive”; veteran battery researcher Jeff Dahn at Dalhousie University in Halifax, Canada, calls it “impossible”. The energy density of rechargeable batteries has risen only sixfold since the early lead–nickel rechargeables of the 1900s. But, says Dahn, the JCESR’s target focuses attention on technologies that will be crucial in helping the world to switch to renewable energy sources — storing up solar energy for night-time or a rainy day, for example. And the US hub is far from alone. Many research teams and companies in Asia, the Americas and Europe are looking beyond Li-ion, and are pursuing strategies that may topple it from its throne.

Lose the dead weight
Chemical engineer Elton Cairns suspected he had tamed a promising-but-wild battery chemistry early last year, when his coin-sized cells were still going strong even after a few months of continual draining and recharging. By July, his cells at the Lawrence Berkeley National Laboratory in Berkeley, California, had cycled 1,500 times and had lost only half of their capacity1 — a performance roughly on a par with the best Li-ion batteries.
His batteries are based on lithium–sulphur (Li–S) technology, which uses extremely cheap materials and in theory can pack in five times more energy by weight than Li-ion (in practice, researchers suspect, it will probably be only twice as much). Li–S batteries were first posited 40 years ago, but researchers could not get them to survive past about 100 cycles. Now, many think that the devices are the technology closest to becoming a commercially viable successor to Li-ion.
One of Li–S’s main advantages, says Cairns, is that it gets rid of the “dead weight” in a Li-ion battery. Inside a typical Li-ion cell, space is taken up by a layered graphite electrode that does little more than host lithium ions. These ions flow through a charge-carrying liquid electrolyte into a layered metal oxide electrode. As with all batteries, current is generated because electrons must flow around an outside circuit to balance the charges. To recharge the battery, a voltage is applied to reverse the electron flow, which also drives the lithium ions back.
In a Li–S battery, the graphite is replaced by a sliver of pure lithium metal that does double duty as both the electrode and the supplier of lithium ions: it shrinks as the battery runs, and reforms when the battery is recharged. And the metal oxide is replaced by cheaper, lighter sulphur that can really pack the lithium in: each sulphur atom bonds to two lithium atoms, whereas it takes more than one metal atom to bond to just one lithium. All of that creates a distinct weight and cost advantage for Li–S technology.
But the reaction between lithium and sulphur causes a problem. As the battery is charged and discharged, soluble Li–S compounds can seep into the electrolyte, degrading the electrodes so that the battery loses charge and the cell gums up. To prevent this, Cairns uses tricks made possible by advances in nanotechnology and electrolyte chemistry — including adulterating his sulphur electrode with graphene oxide binders, and using specially designed electrolytes that do not dissolve lithium and sulphur so much. Cairns predicts that a commercial-sized cell could achieve an energy-density of around 500 Wh kg1. Other labs are reporting similar results, he says.

Some researchers doubt that the academic cheer will translate into commercial success. Laboratories often use low proportions of sulphur and lots of electrolyte, which is relatively easy to work with but does not create an energy-dense battery. Bumping up the sulphur and decreasing the electrolyte makes the cell more likely to gum up, says Steve Visco, who has spent more than 20 years working on Li–S at battery firm PolyPlus in Berkeley, just 5 kilometres west of Cairns’ lab. Making a cheap commercial cell that works over a range of temperatures will also be hard, he says.
At least one company stands by Li–S’s prospects: Oxis Energy in Abingdon, UK. It says it has run large cells for an impressive 900 cycles, at energy densities that match current Li-ion cells. Oxis is working with Lotus Engineering, headquartered in Ann Arbor, Michigan, on a project to reach 400 Wh kg1 by 2016 for an electric vehicle.
Pack more punch per ion
As the world’s lightest metal, lithium provides a huge weight advantage. But some researchers argue that the next generation of cells should switch to heavier elements such as magnesium. Unlike lithium ions, which can carry only one electrical charge each, doubly charged magnesium ions shuttle two at a time — instantly multiplying the electrical energy that can be released for the same volume.
Magnesium comes with its own challenge, however: whereas lithium zips through electrolytes and electrodes, magnesium with its two charges moves as if through treacle.
Peter Chupas, a battery researcher at Argonne National Laboratory who is working with the JCESR, is shooting high-energy X-rays at magnesium in various electrolytes to investigate why it experiences so much drag. So far, he and his colleagues have found that magnesium exerts a strong pull on oxygen atoms in any surrounding solvent, attracting clusters of solvent molecules that make it bulkier. That kind of basic research is key to creating a better battery, but it is not usually done by industry, says Crabtree. “The typical R&D operation operates on trial and error, not fundamental research,” he says. This, he says, is where JCESR is bringing an advantage to the field.
Materials scientist Kristin Persson at Lawrence Berkeley is using a supercomputer to simulate the innards of possible new batteries, trying to find a combination of electrodes and electrolytes that will allow magnesium to pass through more easily. “Right now, we are crunching through around 2,000 different electrolytes,” she says.
Persson and Gerbrand Ceder, a materials scientist at the Massachusetts Institute of Technology in Cambridge, founded a company to develop these higher-charge-carrying batteries. Pellion Technologies, based in Cambridge, is tight-lipped about its results; it has published only one paper about electrolytes2. A spate of patents published in late 2013 hint that the company is developing more-open electrode structures to help the magnesium ions to flow. Major electronics firms such as Toyota, LG, Samsung and Hitachi are also working on such cells, releasing little information beyond occasional teasers.
As companies jostle in secret, Persson continues to run through what she calls the “electrolyte genome”. The sifting-by-supercomputer approach could also help the search for batteries made with other multiple-charge-carrying (or ‘multivalent’) metals, such as aluminium and calcium. Ceder urges patience, pointing out that research into Li-ion battery chemistry has enjoyed a 40-year head start. “We have so little information about multivalent ions,” he says.
Make batteries that breathe
Winfried Wilcke, who describes himself as “an extremely happy owner of a Tesla S” electric car, credits the vehicle with changing his mind about battery-research priorities.
Five years ago, Wilcke, who heads IBM’s nanoscience and technology division in San Jose, California, launched a project to develop a car battery with an 800-kilometre range. At the start, he focused on the theoretical ultimate in energy-dense electrochemical storage: the oxidation of lithium with oxygen drawn from the air. Such ‘breathing’ batteries have a huge weight advantage over other types, because they do not have to carry around one of their main ingredients. A lithium–oxygen (Li–O) battery can, in theory, store energy as densely as a petrol engine — more than ten times better than today’s car battery packs.
But after driving more than 22,000 kilometres in his electric roadster, Wilcke is happy with the 400-kilometre range that its battery already provides. The real problem, he says, is money: battery packs for electric cars cost more than $500 kWh1. “What’s holding back the mass acceptance of electric cars is really the price rather than the energy density,” he says. So Wilcke now favours a cheaper breathing battery based on sodium. Theory predicts that sodium–oxygen (Na–O) batteries could provide only half the energy density of Li–O, but that is still five times better than Li-ion batteries. And sodium is cheaper than lithium, so Na–O might, Wilcke hopes, get closer to the $100-kWh1 goal that the JCESR and others have set for affordability.
Wilcke’s change of heart was undoubtedly influenced by the fact that many have given up hope on Li–O. Researchers who have tried to make it work over the past 20 years have wrestled with unwanted side reactions: carbon in the electrolyte and electrode material react with the lithium and oxygen to form lithium carbonate, so that in every cycle, some 5–10% of the battery capacity is lost. After 50 cycles or so, the battery suffocates. “The bottom line is that Li–O has zero chance for vehicles,” says Stanley Whittingham at Binghamton University in New York, who invented the concept of Li-ion batteries in the 1970s and still focuses on squeezing the best performance out of them. Researchers hoping to resuscitate Li–O include Peter Bruce, a chemist at the University of St Andrews, UK. “We are closer to what’s needed than we were a few years ago,” he argues. But many consider it a lost cause.
Wilcke took an interest in the sodium breathing battery last year, following a surprising discovery by a team including Jürgen Janek and Philipp Adelhelm at the Justus-Liebig University of Giessen in Germany. They found that a Na–O battery recharges more efficiently than Li–O, without complicating side-reactions3. “We tried it and were pretty stunned,” says Wilcke. Plus, he says, it works with cheap electrodes and electrolytes. Janek says that his team has now shown that its battery can work reversibly for at least 100 cycles — not bad for the early days of the technology. Chemicals giant BASF is now working with them.
Dahn, for one, is not convinced. Debate rages about whether breathing batteries will require heavy filtering equipment to extract oxygen from the air, which would cut down or even eliminate their energy-per-weight advantage. “Na–O is just the latest craze,” says Dahn. But Wilcke is willing to bet otherwise.
Go big for the grid
Donald Sadoway’s vision of the future battery looks like a smelting plant: he envisions crates the size of shipping containers, each holding 20 refrigerator-sized steel blocks containing litres of molten metals and salts heated to 500 °C.
Such batteries could never fit in a car, and cannot beat Li-ion on measures such as energy stored per unit weight. But when it comes to storing energy for the electricity grid — or other non-portable applications — size does not matter. Instead of a small, light battery that packs a powerful punch, what people need is a battery that cheaply bottles and releases small-to-large amounts of electricity without much maintenance. The JCESR wants such batteries to last for 7,000 cycles, or about 20 years.
“The field is wide open,” says Ceder. Grid suppliers have used banks of cheap, old-fashioned lead–acid batteries, for example, or stacks of Li-ion. A dizzying array of other chemistries are in development, including zinc–air and sodium-ion. Most technologies are doing well to cost five times as much as the JCESR’s $100-kWh1 target.
Sadoway, a materials chemist at the Massachusetts Institute of Technology, is developing an alternative with two layers of molten metal as electrodes, separated by their different densities and by a layer of molten-salt electrolyte. The metal layers swell or shrink as ions pass between them, storing or releasing energy. Because everything is liquid, there is nothing that could crack after thousands of cycles, as solid electrodes might.
Crabtree, Dahn and other researchers worry about the energy needed to keep the components molten. But Sadoway says that the charging and discharging processes produce enough heat on their own. His company — Ambri in Marlborough, Massachusetts — plans to install test batteries in Hawaii and at a military base on Cape Cod, Massachusetts, this year, each supplying tens of kilowatt-hours.
Other research groups are pursuing less-radical flow batteries, in which the fuel consists of two liquids that pass ions to each other through a membrane. The liquids can be kept in tanks outside the battery and pumped in to flow past each other when needed, so it is possible to store larger amounts of energy indefinitely simply by using bigger tanks. But they do need pumps and valves, which Sadoway says will require maintenance.
Commercial flow batteries use vanadium ions in the liquid on both sides of the barrier. But vanadium and the membranes are expensive: the world’s largest flow battery, installed at a wind farm in China, probably costs $1,000 kWh1, estimates Huamin Zhang at the Chinese Academy of Sciences’ Dalian Institute of Chemical Physics. “The cost of vanadium just kills you,” says Michael Aziz, a materials scientist at Harvard University in Cambridge, Massachusetts.
In January this year, a team including Aziz announced4 that cheap organic chemicals called quinones could be used in a flow battery, partnered to a standard liquid electrode such as bromine. Aziz has cycled his system more than 100 times and it is still running strong. He hopes that he can get such batteries below the magic $100 kWh1, but “this is a toy in a fume hood in a laboratory right now”, he says. “There is no way to know the true cost until you are mass-producing it.”
Crabtree calls the work “promising” and says that the JCESR is also looking at organic chemicals for flow batteries. Another option it is pursuing is to use liquid Li–S and solid lithium in a sort of half-flow battery.
“It’s early days: people are looking at really oddball systems, and everyone’s trying to figure out how to get the lifetime up and the costs down,” says Dahn. The JCESR, for one, is hoping that basic research can fill in the gaps and make these technologies work. “The beyond-lithium-ion space is rich with opportunity,” says Crabtree, “and mostly unexplored.”
You can return to the main Market News page, or press the Back button on your browser.

