Scientists use Visible Light to Break Down Carbon Dioxide
 We all are familiar with the effects of carbon dioxide on our environment. Carbon dioxide is responsible for causing the greenhouse effect. If scientists can breakdown this gas into other form it would lead us to reduce the concentration of this gas into environment substantially. It would mean dealing with the root cause of the problem. Now scientists are trying out to get hold of an organism which could help in the breakdown of carbon dioxide.
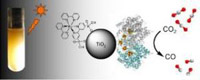
We all are familiar with the effects of carbon dioxide on our environment. Carbon dioxide is responsible for causing the greenhouse effect. If scientists can breakdown this gas into other form it would lead us to reduce the concentration of this gas into environment substantially. It would mean dealing with the root cause of the problem. Now scientists are trying out to get hold of an organism which could help in the breakdown of carbon dioxide.Steve Ragsdale who is a biological chemist from University of Michigan; he and his research assistants Elizabeth Pierce and Fraser Armstrong along with his team from the University of Oxford in the U.K. are working towards breaking down carbon dioxide into benevolent form. It is being said that they have devised means to efficiently turn carbon dioxide into carbon monoxide with the help of visible light, such as sunlight. In this collaboration between Ann Arbor and Oxford they have divided their work. Ragsdale’s laboratory at the University of Michigan Medical School is performing the biochemistry and microbiology experiments. Armstrong’s lab is looking after the physical- and photochemical applications. Ragsdale’s lab received funding from the National Institute of General Medical Sciences at the National Institutes of Health. They have published their findings in the online edition of the Journal of the American Chemical Society.
If scientists can successfully convert carbon dioxide into some useful compound commercially using little energy then we can effectively deal with the ill effects of greenhouse. Some organisms are engaged in this work. Ragsdale tries to explain this phenomenon, “This is a first step in showing it’s possible, and imagine microbes doing something similar. I don’t know of any organism that uses light energy to activate carbon dioxide and reduce it to carbon monoxide, but I can imagine either finding an organism that can do it, or genetically engineering one to channel light energy to coax it to do that.”
Ragsdale and his team have used an enzyme-modified titanium oxide successfully to get carbon dioxide’s electrons excited. This helps in jumping the electrons into the enzyme. After this enzyme starts its catalyst activity and helps in the reduction of carbon dioxide to carbon monoxide. A photosensitizer is attached to the titanium. This photosensitizer helps the utilization of visible light for the process. The enzyme is more forceful than other catalysts. Due to its robustness the whole process can be repeated. But they have taken precautions so it doesn’t come near oxygen.
Armstrong explains the process, “By using this enzyme, you put it into a solution that contains titanium dioxide in the presence of a photosensitizer. We looked for a way that seems like nature’s way of doing it, which is more efficient.” Armstrong says further, that “essentially it shows what is possible were we to be able to mass-produce a catalyst with such properties.”
By performing this experiment scientists got carbon monoxide as a desirable chemical. Carbon mono oxide contains noteworthy fuel value. With the help of catalysts it can be converted into hydrocarbons or methanol. They are liquid fuels. This carbon monoxide is useful in producing electricity or hydrogen. But we have to tread with caution. Carbon monoxide acts as toxin for animals. It is useful as source of energy for microbes. So its risk factor has to be given due consideration.
You can return to the main Market News page, or press the Back button on your browser.

