Chemcycling: what it is and how it is changing our opportunities for circularity
The chemical industry hopes to transform by using chemical recycling to reduce these carbon emissions and enable the circular use of materials.
It’s only recently that chemcycling of plastic waste and scrap tires has been considered a serious recycling option and necessary technologies have begun operations on an industrial scale.
In the EU, only 32.5 percent of the collected plastic waste is recycled, and the rest is landfilled or incinerated for energy recovery, leading to carbon emissions, pollution, and wasting of valuable material.
The chemical industry hopes to transform by using chemical recycling to reduce these carbon emissions and enable the circular use of materials.
What is chemical recycling?
Chemical recycling, or chemcycling, techniques break the bonds that form polymer chains in plastic waste to produce monomers, the building blocks of plastics and tires. The monomers from chemical recycling are secondary raw materials that can serve as feedstocks to make the same or similar plastics as from virgin feedstocks. These plastics have similar properties as products made from fossil fuel feedstocks.
The chemcycling process differs from mechanical recycling, which only breaks down plastics physically.
Chemcycling technologies can easily recycle mixed plastics. This higher tolerance of chemical recycling for mixed plastic waste streams is an advantage over mechanical recycling, which can process only sorted plastic. Chemcycling can also recycle the various types of plastics that are difficult to recycle mechanically.
About 25.8 MT of plastic waste is generated in the EU. Of the collected plastic waste, only 30 percent is recycled (half of what happens in other countries), 39 percent is incinerated, and 31 percent is landfilled. Hence, chemcycling can be a vital complement to mechanical recycling methods to treat waste currently being landfilled or burned.
Chemcycling can turn problematic plastic waste into a resource. The chemical industry has already shown it’s possible to manufacture consumer products using secondary raw materials produced by chemcycling. These include food packaging, carpets, mattresses, refrigerator parts, and car dashboards.
Why is chemical recycling important?
Chemcycling can make the plastics industry circular if implemented on a large scale. With the cooperation of all stakeholders, chemcycling could close the material loop in plastic production, reducing the extraction and use of fossil fuels needed to produce plastics by producing virgin-like polymers.
Natural gas and crude oil provide nearly 99 percent of the raw materials for plastic production, according to Neilsen et al. 2019. They also state that 8 to 9 percent of global fossil fuel consumption is for plastic production. About 4 to 5 percent of the oil and gas serve as raw materials and 3 to 4 percent as fuel for plastic production.
As global plastic production continues to increase by 5 percent annually, replacing even a percentage of virgin polymer with recovered polymers can provide several benefits.
Countries can reduce their reliance on sizable fossil fuel imports using their carbon-rich plastic wastes as a resource instead of exporting them; the benefits of a circular economy will follow this. By encouraging the recycling of plastics within a region, the chemical industry can enable decentralized, dispersed, and sustainable production of secondary feedstocks, fuel, and plastics. As a result, local job opportunities and the economy are boosted.
Chemcycling can reduce plastic leakage into the environment and the production of microplastic particles polluting water bodies and oceans. Plastic is more likely to be collected and passed on for recycling if it’s considered a resource.
Landfilling and incineration of non-recyclable, mixed, and contaminated plastic wastes should also be reduced. According to a scientific study, landfilling produces 253g of CO2 per kg of plastic. Incineration has a much higher carbon footprint. The same study reports that, on average, 673-4605g of CO2 is emitted per kg of plastic waste that is burnt, though using efficient systems can reduce this carbon footprint.
According to Jeswani et al. 2021, chemcycling has a 50 percent lower carbon footprint and energy use in the lifecycle than incineration. Therefore, chemcycling is a better method of using plastic waste to produce energy or fuels than incineration. Diverting plastic wastes to recycling from landfilling and burning can reduce emissions.
Moreover, plastics produced from monomers produced through pyrolysis, one of the chemical recycling methods, have a small carbon footprint. The carbon emissions are lower by 2.3 t CO2 eq./t of plastic than virgin plastic produced from naphtha. There is also 38 percent less energy use in secondary plastic production than in virgin plastics, according to Jeswani et al. 2021.
The carbon footprint of mechanical and chemical recycling are similar. However, depending on the area and technique used, pyrolysis can have a higher environmental impact than mechanical recycling and incineration. Overall, pyrolysis is the preferable option due to its lower carbon footprint and resource extraction. The European Commission even recommends the collection of marine waste to treat it through chemical recycling.
How does chemical recycling work?
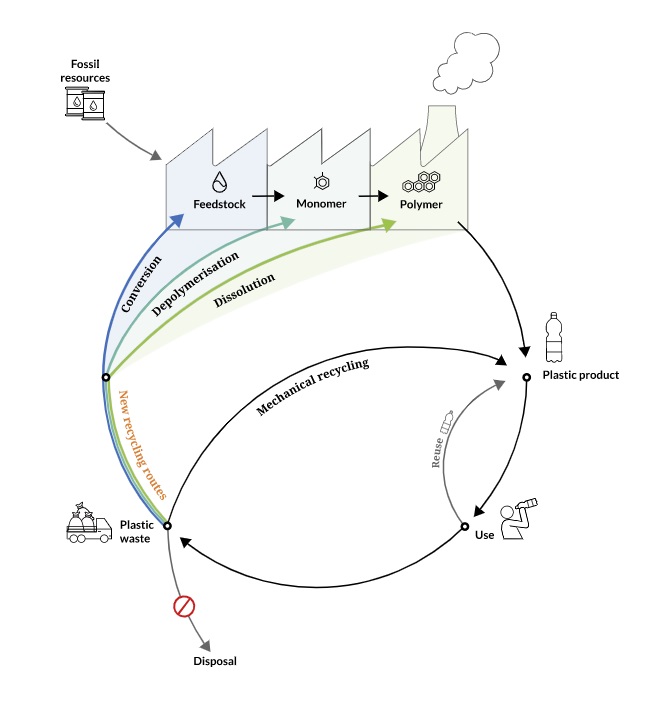
There are several theoretically possible chemcycling technologies. However, only a few operate on a large scale. According to Vollmer et al. 2020, three main categories of technologies are currently used for chemical recycling depending on the catalyst, solvent, and products: depolymerisation, dissolution, and thermochemical processes.
- Depolymerisation – This technique breaks polymer bonds to give monomers and oligomers. Other byproducts can be formed due to associated reactions or interactions with a medium.
- Dissolution – This process uses various solvents to dissolve the plastics and separate polymers from pigments and additives. Different solvents are selectively used for extracting target polymers. Hence breakdown of mixed plastics can be inefficient and result in less separation of the targeted polymer. Several of these methods are in the research or development stages. Operational plants for chemcycling plastics are few, scattered worldwide, and based on conventional pyrolysis, catalytic pyrolysis, and gasification. According to Vollmer et al. 2022, scaling pilot projects to large-scale operations has so far been hindered by a lack of commercial viability, logistics, and available waste streams.
- Thermochemical processes – These processes use heat to break down the polymer bonds and give monomers in an inert or reactive atmosphere. The techniques in this category are pyrolysis, hydrolysis, solvolysis, and gasification.
- Pyrolysis or conversion is by far the most widely used chemical recycling technique. In conventional pyrolysis, the plastics are heated in an inert atmosphere, achieved by using nitrogen, to yield gas, liquids, and waxes. These feedstocks can act as raw materials for monomer production. Liquefaction is pyrolysis conducted under pressure. Catalytic cracking is pyrolysis that uses a catalyst. Plasma pyrolysis and microwave pyrolysis techniques are also being researched.
- Hydropyrolysis or Hydrocracking involves breaking down bonds thermally in a hydrogen atmosphere.
- Solvolysis processes are named after the cleavage agent that breaks carbon bonds in a thermochemical process to produce monomers. For example, hydrolysis uses water. Other solvolysis processes are alcoholysis, phosphorolysis, ammonolysis, and aminolysis.
- Gasification involves heating solid plastic wastes and a gasifying agent to produce a mixture of syngas and hydrocarbon wastes.
Chemical recycling at Klean Industries
Klean industries have been actively involved in chemical recycling for over 6 decades and offer industries more solutions to reduce waste and reuse materials.
Klean Industries has many references of our technologies operating commercially to undertake chemcycling specifically to treat end-of-life tires made partially of synthetic rubber produced from plastic polymers. The patented pyrolysis process can produce recovered tire pyrolysis oil (rFO), recovered gas (rSG), recovered Carbon Black (rCB), and recovered steel (rSW).
The recovered oil can be used as feedstock for the petrochemical industry, and in the carbon black industry and gas can be used as fuel. The rCB is an excellent alternative to fossil-fuel-based virgin Carbon Black for many products like tires, non-tire rubber products, paints, and plastic items. Klean Industries’ pyrolysis products can help the tire and several other industries join the circular economy.
Get in touch to learn more about our sustainable solutions » GO.
If you liked reading this article, we recommend the following content:
You can return to the main Market News page, or press the Back button on your browser.

