Strange airborne yeast threatens human health in US and Canada
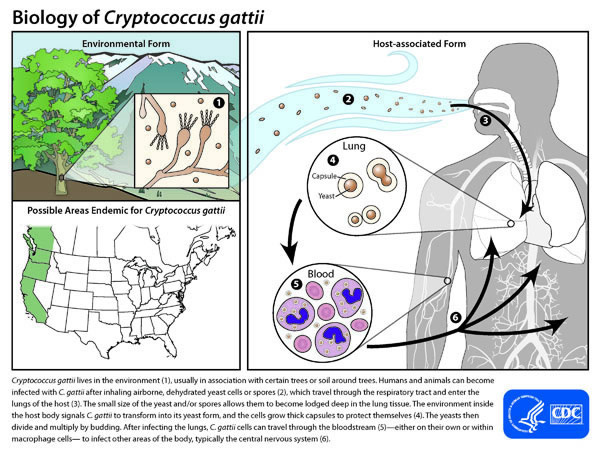
Cryptococcus gattii lives in the environment (1), usually in association with certain trees or soil around trees. Humans and animals can become infected with C. gattii after inhaling airborne, dehydrated yeast cells or spores (2), which travel through the respiratory tract and enter the lungs of the host (3). The small size of the yeast and/or spores allows them to become lodged deep in the lung tissue. The environment inside the host body signals C. gattii to transform into its yeast form, and the cells grow thick capsules to protect themselves (4). The yeasts then divide and multiply by budding. After infecting the lungs, C. gattii cells can travel through the bloodstream (5)—either on their own or within macrophage cells— to infect other areas of the body, typically the central nervous system (6).
Canadian scientists were baffled when in 2001, dead porpoises began washing up on the southeastern shore of Vancouver Island. Autopsies of the animals revealed their lungs were packed with flower-like tumors that left barely enough room for air.
Soon cats and dogs on the island started having trouble breathing, and people began coming to doctors with strange symptoms. They coughed constantly, had headaches and night sweats. X-rays showed lung and brain nodules, but the culprit wasn’t cancer. When doctors biopsied the tissue, they discovered an alien strain of yeast.
Cryptococcus gattii as it was named, was once limited to the tropics and sub-tropics, but it suddenly jumped ship around 2000 and started appearing in a new and deadly form. Since then it’s spread to mainland British Columbia, Washington and Oregon, and it shows no sign of stopping.
In 2001 dead porpoises with yeast-packed lungs washed up on the southeastern shore of Vancouver Island in British Columbia. The bloated organs were several times normal weight, with barely any room for air. The island’s veterinarians had never seen anything like it. Cats and dogs there were having trouble breathing, too. In cats, the disease could cause a particularly gruesome symptom: weeping holes, produced when a yeast infection ate its way through the skull. At the same time, a few people on the island, located off Canada’s Pacific Coast, also began falling ill with an unknown respiratory malady. They coughed constantly, their energy sapped, their sleep stolen. Chest x-rays revealed ominous lung or brain nodules. Biopsied tissue, however, proved the culprit to be not cancer but yeast.
Despite their varying symptoms, the pets, porpoises and humans all shared a single tormenter: Cryptococcus gattii. This fungus had never been seen on the island before, nor was it known to survive outside the tropics and subtropics. Now it was present in the environment, although no one knew where it had come from or how long it had been there. Most worryingly, no one knew how many would be sickened or how far the upstart yeast might travel.
Healthy humans are strangely impervious to fatal fungi. It usually takes something like a shot in the spine with a contaminated drug to give fungi the necessary upper hand. Sure, fungi can be maddening skin irritants, but when was the last time you heard that someone with a normal immune system had died of a fungus? It does happen, as sufferers of Valley Fever, Histoplasmosis, and a handful of other diseases can attest, but it is rare.
So it came as a big surprise, when, in 2001, the public health authorities in British Columbia realized they had something seemingly unprecedented on their hands: a growing outbreak of a lethal fungal pathogen capable of infecting healthy people and never before been seen on the island — and suddenly, inexplicably, four times more virulent.
You can find out what happened next in my first feature story for Scientific American magazine, complete with gorgeous graphics by Sci Am’s Art Department, in the December 2012 print issue (Vive la Print!). Or you can read it online here. I also wrote an Online Extra that looks at how a fungus that ordinarily lives on trees and soil is able to make itself at home inside a human (or porpoise or ferret).
Sudden fungal outbreaks have long been routine among plants, and more recently, animals. A recent outbreak among humans in the Pacific Northwest raises the disturbing prospect that we are not immune either. The mystery of this outbreak’s origins is detailed in “Strange Fungi Now Stalk Healthy People” in the December issue of Scientific American.
The outbreak is ongoing but, in spite of appearances, Cryptococcus gattii doesn’t exist to plague us. The fungus prefers to live in soil and on trees, where it subsists quite happily on decaying matter. So how can an organism that seems to enjoy a full and rich life on plants and dirt possibly find itself suited to living inside humans? The answer, it turns out, may be an accident of evolution.
Life in the wild is not all sunshine and rotting roses for C. gattii. “Microorganisms are in a constant fight for territory, for food sources, for their place in that microbial community,” says Karen Bartlett of the University of British Columbia, an expert in the behavior of biological aerosols. Yeasts have many predators, and formidable among them are amoebas. These protists ooze their way through the soil and water of the world, engulfing and digesting tiny prey. To prevent amoebic annihilation, Cryptococcus species have evolved mechanisms to elude their would-be predators, such as a drying- and digestion-resistant coat, UV-protective pigments and the ability to survive being swallowed by predators.
Those same mechanisms allow yeast to evade a type of human immune cell that looks and acts just like an amoeba (similar cells are also found in other animals). We call them macrophages. Macrophages, which may share evolutionary roots with free-living amoebas, do virtually the same job in humans that amoebas do in the environment: they crawl around eating things. In our case, those things are bits of junk and microbes, which they ingest and kill with digestive enzymes—just like wild amoebas. “If you didn’t know the difference, you’d think that they were amoebas,” Bartlett says.
And apparently, neither does Cryptococcus. In our lungs macrophages scour the surface, mopping up the many foreign objects that land but don’t belong there. In susceptible people and animals Cryptococcus species are just as skillful in duping macrophages as they are their soil attackers. And they use the same methods, at least in the lab.
C. gattii not only can kill macrophages, they can also hide inside them. If ingested, the fungal cells resist digestion while hiding from antibodies, T cells, and other immune system components, effectively converting a macrophage into a microbial Trojan horse. Macrophages travel extensively through the body and can cross the blood–brain barrier. If a yeast cells finds its way from the lung to the brain via a phage or other routes, “that’s very bad news,” Bartlett says, “because once it gets into the central nervous system it’s in heaven. It has all of the sugars it wants to be able to rapidly proliferate.” When Cryptococcus kills it’s generally because such a brain infection has happened.
There are other reasons Cryptococcus has an easy job when it infects us. Unlike the vast majority of fungi, it can survive at 37 degrees Celsius—human body temperature. And it has a tough polysaccharide coat that helps prevent it from drying out in the environment but also helps protect it from macrophages. Finally, its exterior contains melanin, the same pigment that colors human skin, which both protects it from UV radiation as well as inhibits the digestive action of macrophages. “All of these things are protective mechanisms that have allowed it to become established in the environment,” Bartlett says, “and unfortunately those same protective mechanisms make it a pathogen for us.”
Amazingly, this is not an isolated phenomenon. Legionella pneumophila, the bacterial cause of Legionnaires’ disease, lives symbiotically inside wild aquatic amoebas and similarly mistakenly infects human macrophages when victims inhale it. Arturo Casadevall, chair of the Department of Microbiology and Immunology and director of the Center for Immunological Sciences at the Albert Einstein College of Medicine, who has been studying Cryptococcus for over 20 years, has compared the phenomenon with a card game where soil microbes are playing for survival, but by chance, a few hands confer “accidental virulence” on other hosts.
“Virulence is not their business,” Casadevall says. “Their business is survival. But the same pressures that are allowing them to survive results in traits that gives them capacity to survive in mammals.”
You can return to the main Market News page, or press the Back button on your browser.

