New Hydrogen Storage Method
 Hydrogen is an extremely environment friendly fuel as when it burns it releases only water vapor into the atmosphere, but the problem is that it is not easy to store it because it needs to be stored like other compressed gases. A new solid may solve the problem. A nonreactive noble gas called xenon combined with hydrogen and other massive pressure gives rise to a solid that can be later used to store hydrogen fuel. The research paper is published in the November 22, 2009, advanced online publication of Nature Chemistry. The discovery initiates a new line of materials that which could render impetus to new hydrogen technologies.
Hydrogen is an extremely environment friendly fuel as when it burns it releases only water vapor into the atmosphere, but the problem is that it is not easy to store it because it needs to be stored like other compressed gases. A new solid may solve the problem. A nonreactive noble gas called xenon combined with hydrogen and other massive pressure gives rise to a solid that can be later used to store hydrogen fuel. The research paper is published in the November 22, 2009, advanced online publication of Nature Chemistry. The discovery initiates a new line of materials that which could render impetus to new hydrogen technologies. Xenon is used as an anesthesia, to preserve biological tissues, and it is also used in lighting. Being a noble gas xenon does not typically react with other elements. Researchers used a diamond anvil device to squeeze together xenon and hydrogen.
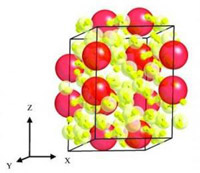
The lead author Maddury Somayazulu, research scientist at Carnegie’s Geophysical Laboratory, explained: “Elements change their configuration when placed under pressure, sort of like passengers readjusting themselves as the elevator becomes full. We subjected a series of gas mixtures of xenon in combination with hydrogen to high pressures in a diamond anvil cell. At about 41,000 times the pressure at sea level (1 atmosphere), the atoms became arranged in a lattice structure dominated by hydrogen, but interspersed with layers of loosely bonded xenon pairs. When we increased pressure, like tuning a radio, the distances between the xenon pairs changed-the distances contracted to those observed in dense metallic xenon.”
The researchers imaged the compound at different pressures using X-ray diffraction, infrared and Raman spectroscopy. They were really surprised to realize that the response of xenon with the surrounding hydrogen was responsible for the unusual stability and the continuous change in xenon-xenon distances as pressure was adjusted from 41,000 to 255,000 atmospheres.
The researchers were taken off guard by both the structure and stability of this material xenon, according to the lead crystallographer who looked at the changes in electron density at varying pressures using single-crystal diffraction. As electron density from the xenon atoms spreads towards the surrounding hydrogen molecules, it stabilizes the compound and the xenon pairs.
Xenon as a hydrogen carrier is too heavy and expensive but further research in this direction can definitely lead to lighter alternatives. According to Russell Hemley, director of the Geophysical Laboratory and a co-author, “This hydrogen-rich solid represents a new pathway to forming novel hydrogen storage compounds and the new pressure-induced chemistry opens the possibility of synthesizing new energetic materials.”
You can return to the main Market News page, or press the Back button on your browser.

